Metadata
TITLE
Zooplankton abundance in the pelagic region of Lake Kasumigaura (Japan): Monthly data since 1980
AUTHORS
Noriko Takamura*, Megumi Nakagawa* and Takayuki Hanazato**
*Center
for Environmental Biology and Ecosystem Studies, National Institute for
Environmental Studies
**Division of Science for Inland Water
Environment, Institute of Mountain Science, Shinshu University, Suwa, Japan
*Corresponding author: Noriko Takamura
Center for Environmental Biology and Ecosystem Studies, National Institute for
Environmental Studies (NIES)
Email: cebes.data@nies.go.jp
Telephone number: +81-298-50-2471
Fax number: +81-298-50-2577
Address:
National Institute for Environmental Studies, Onogawa 16-2, Tsukuba 305-8506,
Japan
ABSTRACT
Microscopic crustaceans (cladocerans and copepods) and rotifers are the principal zooplankton components of the pelagic food webs in lakes. They play important ecological roles, functioning as essential links between primary producers and planktivorous fish. Individuals zooplankton are important nodes of matter flow in pelagic ecosystems. The zooplankton community structure can provide useful indicators of top-down processes, such as the magnitude or strength of planktivorous predators and the extent of zooplankton grazing. Here we report the abundance of zooplankton taxa (crustaceans and rotifers) that were recorded monthly, from January 1980 to September 2015, at two stations on Lake Kasumigaura, a shallow eutrophic lake that is the second largest lake in Japan. The data include information on 12 copepod species (taxa), 20 cladoceran species (taxa), 40 rotifer species (taxa), and the opossum shrimp (Neomysis intermedia). In the 1980s, the plankton of the lake were characterized by cyanobacterial blooms and the co-dominance of Bosmina and Diaphanosoma in the summer. In addition, the two cladoceran genera, Daphnia galeata and Chydorus sphaericus were often prominent. The plankton profile changed dramatically in the middle (1997–2004) of the present long-term monitoring period, when cyanobacteria disappeared and diatoms became dominant even in the summer; concurrently, only the Diaphanosoma cladocerans were evident. However, in the past 10 years , cyanobacterial blooms, the co-dominance of Bosmina and Diaphanosoma, and D. galeata have re-emerged.
Zooplankton monitoring forms part of the Lake Kasumigaura Long-Term Environmental Monitoring Program, which has been conducted by the National Institute for Environmental Studies (NIES) since 1977. Data on other planktonic components (phytoplankton and the elements of microbial food webs) noted during monitoring and on primary production were published in Takamura and Nakagawa (2012ab; 2016). Lake Kasumigaura is a core site of the Japan Long-term Ecological Research Network, a member of the International Long-term Ecological Research Network. Our quantitative dataset spanning several decades is unique in terms of the work on lakes and the plankton therein, and is freely available. The dataset has been used in ecological and environmental programs, as well as in studies on lake management.
KEYWORDS
- Crustacea
- Rotifera
- Mysida
- Pelagic ecosystem
- Zooplankton
- Long-term monitoring
- Eutrophication
- Lake Kasumigaura
INTRODUCTION
Microscopic crustaceans (cladocerans and copepods) and rotifers are the principal zooplankton components of the pelagic food webs of lakes (Wetzel 1983). Crustaceans are the main food of planktivorous fish, and rotifers are essential food resources, particularly for certain fish juveniles and fry of fish. Most zooplankton species of the pelagic zone are grazers and filterers of seston particles including phytoplankton, protozoa, and bacteria. Therefore, zooplankton play an important ecological role, functioning as an essential link between primary producers and planktivorous fish or even piscivores. As such, individual zooplankton are important nodes of matter flow in the pelagic ecosystem.
Zooplankton community structures (species richness and species composition) are determined, or at least influenced, by broad-scale temporal and spatial factors and within-lake factors. The broad-scale factors include historical biogeographic events, such as glacial influences (Roff et al. 1981; Leibold et al. 2010; Viana et al. 2014), and climatic factors causing changes in the duration of ice cover (Gyllström et al. 2005; Catalan et al. 2009), solar radiation (Pinel-Alloul et al. 2013), and thermocline depth and mixing (Gauthier et al. 2014). A Pinatubo event may also be a climatic factor (Stemberger et al. 1996). Furthermore, the broad-scale factors also include the geomorphological structure of the landscape (Juračka et al. 2016), the water chemistry associated with landscape position (Dodson et al. 2009), lake morphology and size ( Catalan et al. 2009; Dodson et al. 2009 ), watershed productivity (Soininen and Luoto 2012), and events such as forest fires (Pinel-Alloul et al. 1998). Environmental variability in both space and time also significantly affect how species share, resources and successfully co-exist (Shurin et al. 2010).
The within-lake factors affecting zooplankton biomass and/or community structure in pelagic ecosystems have been well-studied in terms of the relationships between predators and their food. Fish predation and the presence of planktivores apparently reduce the body size, size at maturity, offspring size, and the feeding rates of certain large crustacean species (Brooks and Dodson 1965; Vanni 1987a; Gliwicz 1994). On the other hand, the size composition of the crustacean zooplankton community influences planktivorous fish production in waters in which the predator-prey ratio is well balanced (Mills and Schiavone 1982; McQueen et al. 1986). Zooplankton biomass and community structure are constrained by the quantity and quality (e.g., mineral balance, nutritional quality, and edibility) of phytoplankton or seston (Vanni 1987a; Vanni and Lampert 1992; Sterner and Hessen 1994). Conversely, zooplankton grazing behavior changes the species composition of phytoplankton (Vanii 1987b; Sterner 1989; Sarnelle 1992; 1993; Steiner 2003). Furthermore, consumer (fish or zooplankton)-mediated transport of nutrient recycling is also important in terms of phytoplankton growth in a pelagic ecosystem (Vanii 1996; Vanni and Layne 1997; Vanni et al. 1997). In particular, Daphnia is a key large herbivore, efficiently filtering a wide size range of waterborne seston particles (approx. 1–20 µm in diameter) (Geller and Müller 1981), thereby decreasing seston density and increasing water transparency. Therefore, Daphnia induces strong trophic cascades (Carpenter et al. 1985; Brett and Goldman 1996), but requires higher P levels in seston particles, constraining cascading trophic interactions. Thus, the zooplankton community structure reflects the structure of the pelagic food-web, and greatly influences the properties of pelagic ecosystems, including primary production and water quality.
Although chlorophyll a, total phosphorus and suspended solid levels are useful indicators of bottom-up processes such as lake nutrient loading or eutrophication, few indicators are available that allow assessments of top-down processes. The zooplankton community structure reflects the magnitude and/or strength of both planktivorous predators and zooplankton grazing. For example, the mean individual body weight of cladocerans, and the zooplankton:phytoplankton biomass ratio, well reflect the magnitude and strength of zooplankton predators and zooplankton grazing, respectively (Jeppersen et al. 2011). Furthermore, changes in cladoceran size and species profile are useful signals of a regime shift event (an abrupt change in lake water transparency) in work exploring whole-lake manipulation (Pace et al. 2013). Long-term monitoring of lake status has been performed in many Western and Eastern European countries (Jeppesen et al. 2011) and China (Xie & Yang 2000; Shao et al. 2001).
Monitoring of the zooplankton of Lake Kasumigaura by the National Institute for Environmental Studies (NIES) commenced in 1976 (Yasuno et al. 1977; Morishita and Yasuno 1979; Yasuno and Morishita 1981). Zooplankton abundance, seasonal changes, and production in the pelagic area during 1984–1991 have been reported by Hanazato (Hanazato et al. 1984; Hanazato and Yasuno 1985ab; 1987abc; 1988; 1989, Hanazato 1991ab; Hanazato and Aizaki 1991; Hanazato et al. 1991). The zooplankton community of Lake Kasumigaura is composed of small-bodied species, with a greater proportion of rotifers and a smaller proportion of large-bodied cladocerans, typical of eutrophic waters (Gliwicz 2004). In the 1980s, Lake Kasumigaura experienced cyanobacterial blooms evidenced by the co-dominance of Bosmina and Diaphanosoma in summer (Hanazato and Yasuno 1987; Takamura et al. 1987). Apart from the two cladoceran genera, Daphnia galeata and Chydorus sphaericus were often present. The plankton composition changed dramatically in the middle (1997–2004) of the present long-term monitoring period (1980–2015); the cyanobacteria disappeared and diatoms became dominant, even in the summer (Takamura and Nakagawa 2012a); concurrently, the only cladoceran was Diaphanosoma. In the past 10 years, however, cyanobacterial blooms featuring co-dominance of Bosmina and Diaphanosoma, and D. galeata, have returned. Mysids(Neomysis intermedia) are common invertebrate predators and Leptodora kindtii sometimes occurs. The dominant copepods are the calanoid copepod, Eodiaptomus japonicas and the cyclopoid copepod, Cyclops visinus, which are usually abundant in warm and cold seasons, respectively. The dominant rotifers are Keratella cochlearis, Brachionus calyciflorus, Trichocerca spp. and Polyarthra spp. Rotifer numbers, measured using a 44-µm mesh, have peaked twice since May 1987; in 1992–96 and in 2011–15.
Monitoring has been a key component of the Lake Kasumigaura Long-term Environmental Monitoring Program run by the NIES since 1977. The program includes collection of data on water quality, plankton, the benthos, and primary production, and the dataset are included in the database of the program. Lake Kasumigaura is a core site of the Japan Long-term Ecological Research Network (JaLTER), a member of the International Long-term Ecological Research Network (ILTER). Three data papers on phytoplankton species abundance(1978–2012); the densities of the bacteria, picophytoplankton, heterotrophic nanoflagellates, and ciliates (1996–2015); photosynthesis and primary production (1981–2015) in Lake Kasumigaura have been published by Takamura and Nakagawa (2012ab and 2016, respectively). This study reports changes in the abundance of zooplankton species (taxa), which were monitored monthly from January 1980 to September 2015 at two stations on Lake Kasumigaura, a shallow lake that is the second largest in Japan. Lake Kasumigaura may be the only Japanese lake for which zooplankton abundance has been monitored monthly for 35 years. Thus, the dataset is unique in terms of lake and plankton monitoring and continues to be freely available. The information will aid in current and future ecological and environmental research studies.
METADATA
1. Title
Zooplankton abundance in the pelagic region of Lake Kasumigaura (Japan): Monthly data since 1980
2. Identifier
ERDP-2016-06
3. Contributor
A. Dataset owners
The National Institute for Environmental Studies (Japan) and the following individuals provided the data for the database.
| Owners and contact individual (*) | Affiliation | Contact | |||
|---|---|---|---|---|---|
| Address | Phone | Fax | Email address | ||
| Noriko Takamura* | National Institute for Environmental Studies | Onogawa 16-2, Tsukuba 305-8506, Japan | +81-298-50-2471 | +81-298-50-2577 | noriko-t@nies.go.jp |
| Megumi Nakagawa* | +81-298-50-2415 | nakagawa.megumi@nies.go.jp | |||
| Takayuki Hanazato** | |||||
**a research member of National Institute for Environmental Studies from April 1980 to November 1995.
B. Dataset creators
Noriko Takamura, Center for Environmental Biology and Ecosystem Studies, National Institute for Environmental Studies
Megumi Nakagawa, Center for Environmental Biology and Ecosystem Studies, National Institute for Environmental Studies
Takayuki Hanazato, Division of Science for Inland Water Environment, Institute of Mountain Science, Shinshu University, Suwa, Japan
C. Principal investigators
Morihiro Aizaki, Senichi Ebise, Takehiko Fukushima, Toshio Iwakuma, Takayoshi Kawai, Noriko Takamura, Takayuki Hanazato, Yukihiro Nojiri, Masaaki Hosomi, Takanobu Inoue, Hideaki Ozawa, Akira Otsuki, Masayuki Yasuno, Akio Imai, Kazuho Inaba, Noriko Tomioka, Kazuhiro Iwasaki, Ayato Kohzu, Takayuki Satou, Kazuhiro Komatsu, Masami Koshikawa, Kazuo Matsushige, Ryuichiro Shinohara, Shin-ichiro Matsuzaki, Megumi Nakagawa, Ryuhei Ueno, Junko Yamamura and Tomiji Hagiwara performed the field survey.
4. Program
A. Title
Lake Kasumigaura Long-term Environmental Monitoring program
B. Personal
Organization:
National Institute for Environmental Studies
Address:
Onogawa 16-2, Tsukuba 305-8506, Japan
Phone:
+81-298-50-2471 (Voice)
Phone: +81-298-50-2577 (fax)
Web Address:
http://www.nies.go.jp/
C. Funding
National Institute for Environmental Studies, Japan
D. Objectives
Since 1977, the NIES has conducted monthly monitoring of the water quality, plankton/benthos biomass and primary production of Lake Kasumigaura. Monitoring was initiated by a group of lake scientists working at the NIES with the intention of sharing fundamental limnological data. Monitoring has continued for more than three decades, in efforts to promote environmental and ecological studies; the work elucidates long-term changes in lake ecosystems and how lake environments may recover from adverse events.
5. Geographic coverage
A. Geographic description
Nishi-ura of Lake Kasumigaura, Japan
B. Geographical position
Station 3: 36°07.302′ N, 140°22.652′ E (WGS84)
Station 9: 36°02.142′ N, 140°24.222′ E (WGS84)
6. Temporal coverage
A. Commencement
January 1980
B. End
September 2015
7. Taxonomic coverage
The zooplankton data include those on 12 copepod species (taxa), 20 cladoceran species (taxa), 40 rotifer species (taxa), and the opossum shrimp (Neomysis intermedia).
8. Methods
A. Study sites
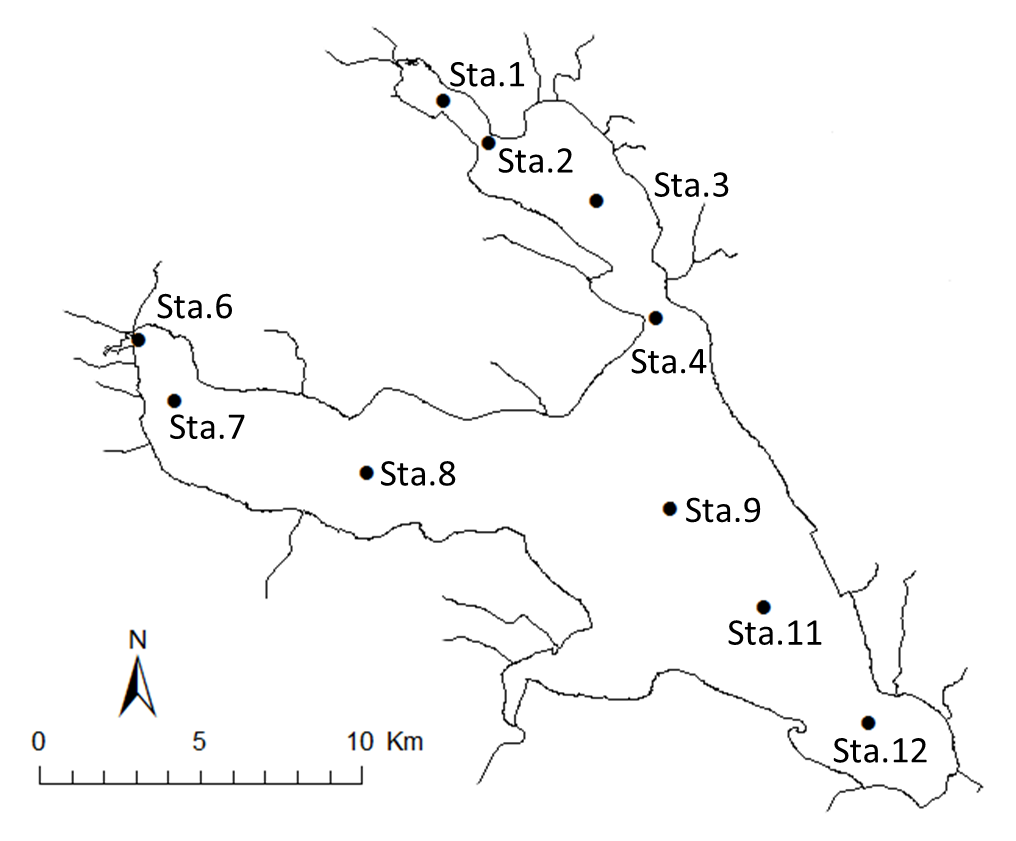
Lake Kasumigaura (Fig. 1) is located approximately 60 km northeast of the Tokyo metropolitan area. The lake is the second largest in Japan (surface area 220 km2, total volume of 0.85 billion m3), and is shallow (mean depth 4 m, maximum depth 7 m), with a catchment area of 2,157 km2. The lake is composed of three parts: Nishi-ura, Kita-ura, and Sotonasaka-ura. Nishi-ura, the largest part of the lake, has a surface area of 167.7 km2 and a total volume of 662 million m3; there are 29 inflows and 1 major outflow.
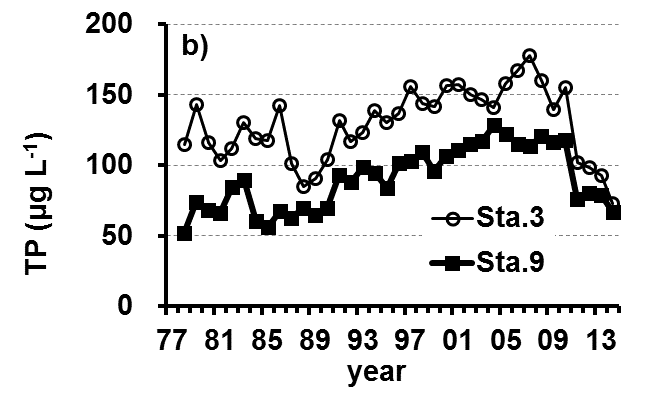
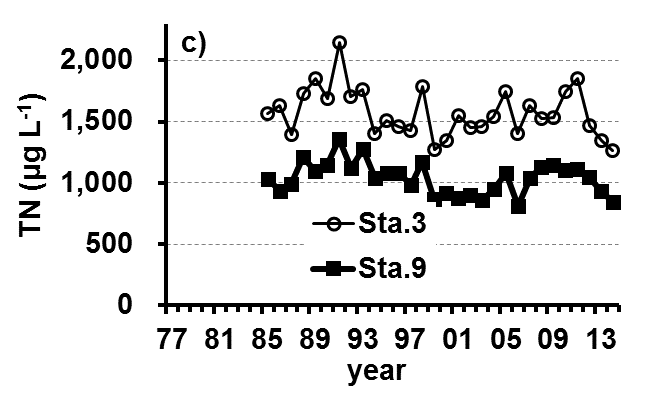
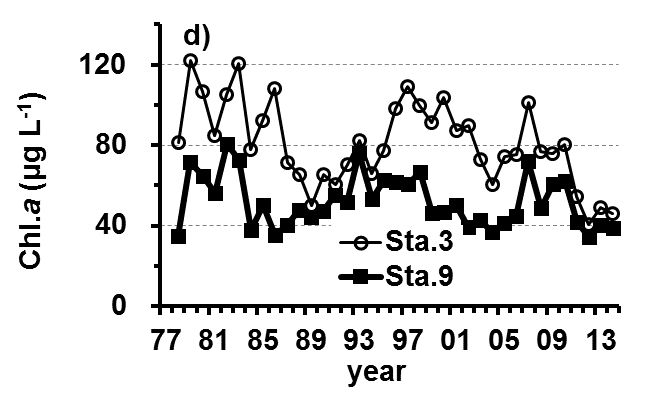
The Lake Kasumigaura Long-term Environmental Monitoring program selected 10 pelagic monitoring sites in Nishi-ura (Fig. 1), and measured particular environmental variables in situ (water temperature, water depth, water transparency, dissolved oxygen level, pH, and downward irradiance); water quality (levels of electrical conductivity (EC), chemical oxygen demand (COD), chlorophyll a (Chl.a), suspended solids (SS), particulate organic carbon (POC), particulate organic nitrogen (PON), total phosphorus (TP), dissolved total phosphorus (DTP), soluble reactive phosphorus (SRP), total nitrogen (TN), dissolved total nitrogen (DTN), ammonium-nitrogen (NH4-N), nitrate and nitrite-nitrogen (NO2-N+NO3-N), Al, B, Ba, Ca, Fe, K, Mg, Mn, Na, Si, Sr, and Cu); plankton (bacteria, HNFs, ciliates, picocyanobacteria, eukaryotic picoplankton, phytoplankton, rotifers, crustacean zooplankton, and mysids); the benthos (chironomids and oligochaetes); and primary production. Water quality, water depth and transparency were measured at every station. Plankton were measured at Station 3 and 9 (Fig. 1). The benthos, primary production and downward irradiance were monitored at Station 3, 7, 9 and 12 (Fig. 1), and other environmental variables (water temperature, dissolved oxygen level, and pH) were measured at Stations 1, 3, 7, 9 and 12 (Fig. 1). The annual means of water transparency and the concentrations of TP, TN, Chl.a, and SS at the two sites from 1978 and 2015 are shown in Figure 2.
Lake changes resulting from recent anthropogenic activities, and the associated environmental problems, were summarized by Takamura (2012). Details of the densities of phytoplankton species (monitored monthly or biweekly since 1978); those of bacteria, picophytoplankton, heterotrophic nanoflagellates, and ciliates (monitored monthly since 1996); and photosynthesis and primary production (measured monthly since 1981) have been reported by Takamura and Nakagawa (2012ab, 2016).
B. Sampling, sample preservation and counting methods
Zooplankton were collected at two stations (3 and 9), with a slightly modified sampling procedure. From January 1980 to March 1981, water samples for zooplankton enumeration were collected using a Van Dorn sampler (6 L) at a depth of 0.5 m. Zooplankton were collected by filtering the sampled water through a NXX13 94 μm mesh net and fixed in 4% (v/v) formalin. In April 1981, use of a column sampler (an acrylic tube 45mm in diameter running from the surface to a depth of 2 m) commenced. Zooplankton were collected using the same mesh net, but the preservation solution was changed from 4% (v/v) formalin (4% (v/v) formalin with 40g sugar L-1) in May 1983. Furthermore, the mesh net was changed to an NXXX25 net (40-μm mesh) in May 1987. This change in mesh size affected the data gathered from April 1981 to April 1987 and from May 1987 to the present. Neither the sampling nor preservation procedures changed between May 1987 and the present. Zooplankton were counted in the laboratory using an inverted microscope (Nikon TMS, Nikon TS10 or Olympus CKX42) at a magnification of 40x or 100x.
Mysids (Neomysis intermedia), important components of the lake food web were collected from August 1984 to the present using a 40 cm-diameter NXX7 200 μm-mesh net. The bottom of the net was first positioned at 1.2 m above the lake bottom, which was not disturbed, and then pulled vertically to the surface. From February 1983 to July 1984, mysids using a 30-cm diameter NXX13 (94 μm) mesh net. The catching efficiency of this small net was only 20% that of the net used subsequently; therefore, mysids numbers in this period should be interpreted cautiously. Mysid sampling and counting were performed in duplicate. The 1980–1982 data for Station 3 were provided by Toda et al. (1983). From January 1990 to March 1996, we did not collect any Neomysis data. The crustacean Leptododa kindtii, was also counted in the samples used to enumerate mysids; this species is larger than the other crustacean zooplankton and found at low densities. The mysids and crustaceans were counted using a binocular microscope (Olympus B061 or Leica MZ16).
C. Taxonomy and systematics
The textbooks used for identification were Mizuno and Takahashi(1991, 2000) and Mizuno(1984) for the Copepoda; Du and Mizuno(1982) for the Cladocera; and Koste(1978) and Sudzuki(1999) for rotifers.
D. Data verification procedures
All of the data were manually digitized and checked for typographical errors. Any unreliable information was scored as an error (please see section 11.D).
9. Data status
A. Latest Update
11 September 2015
Data were collected from January 1980 to September 2015. However, during this period, the sampling, sample preservation and counting methods changed several times (as shown in Section 8. Method B). Data were continuously collected through September 2015 and the database was updated when the new data were verified.
B. Metadata status
The metadata for the relevant period are complete and have been filed and stored with the raw data.
10. Accessibility
A. License and usage rights
1) Acceptable use. The dataset should not be used for any illegal purpose or to violate the rights of others. Dataset usage should be restricted to academic, research, educational, government, or other not-for-profit professional purposes. Data users need to agree to the terms of use for NIES Lake Kasumigaura Database (http://db.cger.nies.go.jp/gem/moni-e/inter/GEMS/database/kasumi/contents/terms.html). Please make sure to contact the data manager of Lake Kasumigaura Database by email (cebes.data@nies.go.jp) before using the dataset.
2) Citation. Data use should appropriately cite the present paper in all of the publications or when citing metadata derived using our data. As our metadata may be updated at any time, the date of the update cited should be shown in the bibliography.
3) Acknowledgements. To support our long-term monitoring, all of the data users should include the following text in any publications using the dataset: “The XXX data are those of the Lake Kasumigaura Long-term Environmental Monitoring Program of the National Institute for Environmental Studies, Japan.”
4) Notification. Data users should notify the nominated Dataset Contact when any derivative work or publication based on or derived from the dataset is contemplated. The Dataset Contact requires two reprints or a PDF file of any publication using dataset details.
5) Collaboration. Data users are strongly encouraged to consider consultation, collaboration, and/or co-authorship with data owners.
6) Disclaimer. In no event shall any author, data owner, or the National Institute for Environmental Studies be liable for loss of profit or for any indirect or incidental damages arising from data use or interpretation.
B. Contact
Dataset Contact
Megumi Nakagawa or Noriko Takamura
Center
for Environmental Biology and Ecosystem Studies, National Institute for
Environmental Studies, Onogawa 16-2, Tsukuba 305-8506, Japan
Telephone number: +81-298-50-2471
Email: cebes.data@nies.go.jp
C. Storage location
The data owners and the National Institute for Environmental Studies store the original data.
11. Data structure
A. Data tables
| Data file name | Description |
|---|---|
| Kasumi_Mysida.csv | Density (individuals of Neomysis intermedia) per cubic meter of lake water collected at Stations 3 and 9. |
| Kasumi_Crustacea.csv | Density (individuals) per liter of lake water of 32 taxa collected at Stations 3 and 9. |
| Kasumi_Rotifer.csv | Density (individuals) per liter of lake water of 40 taxa collected at Stations 3 and 9. |
| TaxonList.csv | The species-wise information table. |
B: Format type
All of the data files are in ASCII text format, and are comma-delimited (using the csv format).
C: Header information
Headers corresponding to the names of variable (see Section 11.D) are included in the first rows of the data files.
D: Definitions of variables
All variables are listed in the order in which they appear in the first rows of the data files. The term “.” indicates that we did not encounter this species during counting. This means that the density of each taxon (species or genus) was less than the minimum numerical value of the range of such values (Table 2). “NA” indicates a missing datum. The definitions of variables are given in Table 1.
| Data file name | Variable name | Variable definition | Range of numerical values |
|---|---|---|---|
| Kasumi_Mysida.csv | Date | Sampling date (yyyy/m/d) | 1980/4/25–2015/9/11 |
| Sta. | Site name | 3, 9 | |
| Taxonomic ID | Identification of taxa (species or genus) | - | |
| Taxa (species or genus) | Scientific names of taxa (species or genus) | - | |
| Density | Density (individuals m-3) | 0.3–4,446 | |
| Kasumi_Crustacea.csv | Date | Sampling date (yyyy/m/d) | 1980/1/23–2015/9/11 |
| Sta. | Site name | 3, 9 | |
| Taxonomic ID | Identification of taxa (species or genus) | - | |
| Taxa(species or genus) | Scientific names of taxa (species or genus) | - | |
| Density | Density (individuals L-1) | 0.001–2,209 | |
| Kasumi_Rotifer.csv | Date | Sampling date (yyyy/m/d) | 1980/1/23–2015/9/11 |
| Sta. | Site name | 3, 9 | |
| Taxonomic ID | Identification of taxa (species or genus) | - | |
| Taxa(species or genus) | Scientific names of taxa (species or genus) | - | |
| Density | Density (individuals L-1) | 0.1–3,077 |
| Data file name | Variable: Taxa (species or genus) | Range of numerical values |
|---|---|---|
| Kasumi_Mysida | Neomysis intermedia | 0.3–4,446 |
| Kasumi_Crustacea | Calanoida (mature) spp. | 0.1–29 |
| Eodiaptomus japonicus | 0.1–82 | |
| Pseudodiaptomus inopinus | 0.1–105 | |
| Clanoida (copepodid) | 0.1–415 | |
| Cyclopoida (mature) spp. | 0.1–67 | |
| Cyclops vicinus | 0.1–27 | |
| Eucyclops spp. | 0.2–1.5 | |
| Mesocyclops sp. | 0.2–30 | |
| Paracyclops fibriatus | 0.2–0.2 | |
| Thermocyclops taihokuensis | 0.3–21 | |
| Cyclopoida (copepodid) | 0.1–623 | |
| nauplius | 0.1–834 | |
| Alona spp. | 0.1–12 | |
| Bosmina fatalis | 0.1–2,209 | |
| Bosmina longirostris | 0.1–1,450 | |
| Bosmina spp. | 0.1–645 | |
| Bosminopsis deitersi | 0.3–8.5 | |
| Ceriodaphnia cornuta | 0.3–241 | |
| Ceriodaphnia reticulata | 1.7–11 | |
| Chydorus sphaericus | 0.2–390 | |
| Daphnia ambigua | 0.3–283 | |
| Daphnia galeata | 0.1–277 | |
| Diaphanosoma brachyurum | 0.1–492 | |
| Disparalona rostrata | 0.2–3.8 | |
| Ilyicryptus sordidus | 0.2–0.2 | |
| Leptodora kindtii | 0.001–2.8 | |
| Leydigia leydigii | 0.4–1.6 | |
| Leydigia sp.1 | 1.2–1.2 | |
| Moina macrocopa | 0.3–22.5 | |
| Moina micrura | 0.1–293 | |
| Pleuroxus hamulatus | 0.1–1.2 | |
| Unknown-cladocera spp. | 2.5–5.0 | |
| Kasumi_Rotifer | Anuraeopsis fissa | 0.6–588 |
| Asplanchna spp. | 0.1–600 | |
| Bdelloidae spp. | 0.3–6.4 | |
| Brachionus angularis | 0.1–575 | |
| Brachionus budapestinensis | 0.3–126 | |
| Brachionus calyciflorus | 0.2–2,016 | |
| Brachionus forficula | 0.3–105 | |
| Brachionus leydigi | 0.5–1.2 | |
| Brachionus quadridentatus | 0.2–69 | |
| Brachionus rubens or urceolaris | 0.1–83 | |
| Collothecidae spp. | 1.1–91 | |
| Colurella spp. | 1.6–1.6 | |
| Conochiloides sp. | 0.3–861 | |
| Conochilus sp. | 0.3–797 | |
| Euchlanis spp. | 0.3–60 | |
| Filinia longiseta | 0.2–250 | |
| Filinia spp. | 0.3–2,026 | |
| Hexarthra mira | 0.3–89 | |
| Keratella cochlearis | 0.1–3,077 | |
| Keratella cochlearis var. micracantha | 1.5–143 | |
| Keratella cochlearis var. tecta | 0.1–2,147 | |
| Keratella quadrata | 0.3–763 | |
| Keratella valga | 0.2–291 | |
| Lecane spp. | 0.5–129 | |
| Lepadella spp. | 0.7–1.2 | |
| Lophocharis spp. | 2.7–117 | |
| Mytilina spp. | 1.4–1.4 | |
| Notholca spp. | 0.3–4.3 | |
| Ploesoma truncatum | 1.6–1.6 | |
| Polyarthra vulgaris | 0.1–1,804 | |
| Polyarthra spp. | 0.1–2,383 | |
| Pompholyx spp. | 0.5–788 | |
| Proales spp. | 4.5–13 | |
| Schizocerca diversicornis | 0.3–110 | |
| Synchaeta spp. | 0.2–674 | |
| Trichocerca captina | 0.1–76 | |
| Trichocerca spp. | 0.1–2,500 | |
| Trichotria spp. | 0.5–8.3 | |
| Unknown-rotifer sp.1 | 0.5–480 | |
| Unknown-rotifer sp.2 | 2.6–795 |
12. Supplementary information
We have deposited the data in the database of the Lake Kasumigaura Long-term Environmental Monitoring Program. This program, run by the NIES, commenced in 1977. Thus, our data are those reported on the Japanese-language website ( http://db.cger.nies.go.jp/gem/inter/GEMS/database/kasumi/index.html ) and on the English-language website ( http://db.cger.nies.go.jp/gem/moni-e/inter/GEMS/database/kasumi/index.html ) (one of the website of NIES, Japan).
13. Acknowledgements
The publication of this data paper was encouraged by J-BON (Japan Biodiversity Observation Network), and was partially supported by JSPS KAKENHI Grant-in-Aid for Scientific Research (A) Number 15H02380 (2015–2018).
14. References
Brett MT, Goldman CR (1996) A meta-analysis of the freshwater trophic cascade. Proc Natl Acad Sci USA 93:7723–6
Brooks JL, Dodson SI (1965) Predation, body size, and composition of plankton. Science 150:28-35
Carpenter SR, Kitchell JF, Hodgson JR (1985) Cascading trophic interactions and lake productivity. BioScience 35:634-639
Catalan J, Barbieri MG, Bartumeus F, Bitušík P, Botev I, Brancelj A, Cogălniceanu D, Manca M, Marchetto A, Ognjanova-Rumenova N, Pla S, Rieradevall M, Sorvari S, Štefková E, Stuchlík E, Ventura M (2009) Ecological thresholds in European alpine lakes. Freshw Biol 54:2494–2517 doi:10.1111/j.1365-2427.2009.02286.x
Dodson SI, Newman AL, Will-Wolf S, Alexander ML, Woodford MP, Van Egeren S (2009) The relationship between zooplankton community structure and lake characteristics in temperate lakes (Northen Wisconsin, USA). J Plankton Res 31:93-100
Du N, Mizuno T (1982) Review of freshwater Cladocera in China and Japan. 209 pp. Tatara-shobo, Yonago (In Japanese)
Gauthier J, Prairie YT, Beisner BE (2014) Thermocline deepening and mixing alter zooplankton phenology, biomass and body size in a whole-lake experiment. Freshw Biol 59:998-1011
Geller W, Müller H (1981) The filtration apparatus of Cladocera: Filter mesh-sizes and their implications on food selectivity. Oecol 4:316-321
Gliwicz ZM (1994) Relative significance of direct and indirect effects of predation by planktivorous fish on zooplankton. Hydrobiol 272:201-210
Gliwicz ZM (2004) Zooplankton. pp461-516, In: O’Sullivan PE, Reynolds CS (eds) The Lakes Handbook volume 1 Limnology and limnetic ecology, Blackwell Scientific Publications, Ocford
Gyllström M, Hansson LA, Jeppesen E, García-Criado F, Gross E, Irvine K, Kairesalo T, Kornijow R, Miracle MR, Nykänen M, Nõges T, Romo S, Stephen D, Van Donk E, Moss B (2005) The role of climate inshaping zooplankton communities of shallow lakes. Limnol Oceanogr 50:2008-2021
Hanazato T, Yasuno M, Iwakuma T, Takamura N (1984) Seasonal changes in the occurrence of Bosmina longirostris and Bosmina fatalis in relation to Microcystis bloom in Lake Kasumigaura. Jpn J Limnol 45:153-157
Hanazato T, Yasuno M (1985a) Population dynamics and production of cladoceran zooplankton in the highly eutrophic Lake Kasumigaura. Hydrobiol 124:13-22
Hanazato T, Yasuno M (1985b) Occurrence of Daphnia ambigua Scourfield in Lake Kasumigaura. Jpn J Limnol 46:212-214
Hanazato T, Yasuno M (1987a) Evaluation of Microcystis as food for zooplankton in a eutrophic lake. Hydrobiol 144:251-259
Hanazato T, Yasuno M (1987b) Experimental studies on competition between Bosmina longirostris and Bosmina fatalis. Hydrobiol 154:189-199
Hanazato T, Yasuno M (1987c) Characteristics of biomass and production of cladoceran zooplankton in Lake Kasumigaura. Jpn J Limnol S45-S57
Hanazato T, Yasuno M (1988) Impact of predation of Neomysis intermedia on a zooplankton community in Lake Kasumigaura. Verh Int Verein Limnol 23:2092-2098
Hanazato T, Yasuno M (1989) Zooplankton community structure driven by vertebrate and invertebrate predators. Oecol 81:450-458
Hanazato T, Aizaki M (1991) Changes in species composition of cladoceran community in Lake Kasumigaura during 1986-1989: Occurrence of Daphnia galeata and its effect on algal biomass. Jpn J Limnol 52:45-55
Hanazato T. (1991a) Species composition of cladoceran community in the highly eutrophic Lake Kasumigaura. Rep Suwa Hydrobiol 7:105-112
Hanazato T. (1991b) Interrelations between Microcystis and Cladocera in the highly eutrophic Lake Kasumigaura, Japan. Int Revue ges Hydrobiol 76:21-36
Hanazato T, Takamura N, Yasuno M (1991) Occurrence of Bosmina longirostris and Bosmina fatalis in enclosures in relation to phytoplankton biomass. Pol Arch Hydrobiol 38:177-182
Jeppesen E, Nõges P, Davidson TA, Haberman J, Nõges T, Blank K, Lauridsen TL, Søndergaard M, Sayer C, Laugaste R, Johansson LS, Bjerring R, Amsinck SL (2011) Zooplankton as indicators in lakes: a scientific-based plea for including zooplankton in the ecological quality assessment of lakes according to the European Water Framework Directive (WFD) Hydrobiol 676:279-297
Juračka PJ, Declerck SAJ, Vondrak D, Beran L, Černy M, Petrusek A (2016) Evaluation of fish communities through assessment zooplankton populations and measures of lake productivity. N Am J Fish Manag 2:14-27
Koste W (1978) Rotatoria. Gebrüder Borntraeger Berlin, Stuttgart.
Leibold MA, Economo EP, Peres-Neto P (2010) Metacommunity phylogenetics: separating the roles of environmental filters and historical biogeography. Ecol Lett 13:1290-1299 doi: 10.1111/j.1461-0248.2010.01523.x
McQueen DJ, Post JR, Mills EL (1986) Trophic relationships in freshwater pelagic ecosystems. Can J Fish Aquat Sci 43:1571-1581
Mills EL, Schiavone A (1982) Evaluation of fish communities through assessment of zooplankton populations and measures of lake productivity. North American J. Fisheries management 2:14-27
Mizuno T (1984) Freshwater Calanoida in Japan. In: Shen CJ, Mizuno T (eds), Chinese/Japanese Freshwater Copepoda. 650pp. Tatara-shobo, Yonago (In Japanese)
Mizuno T, Tahakashi E (1991) An Illustrated Guide to Freshwater Zooplankton in Japan (in Japanese). Tokai University Press, Tokyo
Mizuno T, Tahakashi E (2000) An Illustrated Guide to Freshwater Zooplankton in Japan (in Japanese). Tokai University Press, Tokyo
Morishita M, Yasuno M (1979) Seasonal change and horizontal distribution of zooplankton at Takahamairi Bay in Lake Kasumigaura. Res Rep Natl Inst Environ Stud 6:155-170 (in Japanese)
Pace M, Carpenter SR, Johnson RA, Kurtzweil JT (2013) Zooplankton provide early warnings of a regime shift in a whole lake manipulation. Limnol Oceanogr 58: 525–532 doi:10.4319/lo.2013.58.2.0525
Pinel-Alloul B, André A, Legendre P, Cardilee JA, Patalas K, Salki A (2013) Large-scale geopraphic patterns of diversity and community structure of pelagic crustacean zooplankton in Canadian lakes. Global Ecol Biogeogr 22:784-795
Pinel-Alloul B, Patoine A, Carignan R, Prepas E (1998) Responses of lake zooplankton to natural fire and forest harvesting in the boreal ecozone in Quebec: preliminary study. Annls Limnol 34:401-412
Roff JC, Sprules WG, Carter JCH, Dadswell MJ (1981) The structure of crustacean zooplankton communities in glaciated eastern North America. Can Fish Aquat Sci 38: 1428-1437
Sarnelle O (1992) Nutrient enrichment and grazer effects on phytoplankton in lakes. Ecol 73:551-560
Sarnelle O (1993) Herbivore effects on phytoplankton succession in a eutrophic lake. Ecol Monogr 63:129-149
Shao Z, Xie P, Zhuge Y (2001) Long-term changes of planktonic rotifers in a subtropical Chinese lake dominated by flter-feeding fishes. Freshw Biol 46:973-986
Shurin JB, Winder M, Adrian R, Keller W, Matthews B, Paterson AM, Paterson MJ, Pinel-Alloul B, Rusak JA, Yan ND (2010) Environmental stability and lake zooplankton diversity-contrasting effects of chemical and thermal variability. Ecol Lett 13:453-463 doi:10.1111/j.1461-0248.2009.01438.x
Soininen J, Luoto M (2012) Is catchment productivity a useful predictor of taxa richness in lake plankton communities? Ecol Appl 22:624-633
Steiner CF (2003) Keystone predator effects and grazer control of planktonic primary production. Oikos 101:569-577
Stemberger RS, Herlihy AT, Kugleret DL, Paulsenal SG (1996) Climatic forcing on zooplankton richness in lakes of the northeastern United States. Limnol Oceanogr 41:1093-1101
Sterner RW (1989) The Role of Grazers in Phytoplankton Succession U. Sommer (ed.), Plankton Ecology © Springer-Verlag Berlin Heidelberg New York London Paris Tokyo 107-170
Sterner RW, Hessen DO (1994) Algal nutrient limitation and the nutrition of aquatic herbivores. Ann Rev Ecol Syst 25:1–29
Sudzuki, M (1999) An approach to the identification of the common rotifers. Sanseido, Tokyo
Takamura N, Iwakuma T, Yasuno M (1987) Primary production in Lake Kasumigaura, 1981-1985. Jpn J Limnol S13-S38
Takamura N (2012) The status of biodiversity loss in lakes and ponds in Japan. In: Nakano S, Yahara T, Nakashizuka T (eds) Biodiversity Observation Network in Asia-Pacific region: Towards further development of monitoring activities, Springer, Tokyo
Takamura N., Nakagawa M. (2012a) The densities of bacteria, picophytoplankton, heterotrophic nanoflagellates and ciliates in Lake Kasumigaura (Japan) monitored monthly since 1996. Ecol Res 27:839-839
Takamura N., Nakagawa M. (2012b) Phytoplankton species abundance in Lake Kasumigaura (Japan) monitored monthly or biweekly since 1978. Ecol Res 27:837-837
Takamura N, Nakagawa M (2016) Photosynthesis and primary production in Lake Kasumigaura (Japan) monitored monthly since 1981. Ecol Res 31:287-287
Toda H, Nishizawa S, Takahashi M, Ichimura S (1983) Temperature control on the post-embryonic growth of Neomysis intermedia Czernaiwsky in a hypereutrophic temperate lake. J Plankton Res 5:377-392.
Vanni MJ (1987a) Effects of food availability and fish predation on a zooplankton community. Ecol Monogr 57:61-88
Vanni MJ (1987b) Effects of nutrients and zooplankton size on the structure of a phytoplankton community Ecol 68:624-635
Vanni MJ, Lampert W (1992) Food quality effects on life history traits and fitness in the generalist herbivore Daphnia. Oecol 92:48-57
Vanii MJ (1996) Nutrient Transport and Recycling by Consumers in Lake Food Webs: Implications for Algal Communities. In: Polis GA, Winemiller KO (eds) Food webs, Chapman and Hall, London.
Vanni MJ, Layne CD (1997) Nutrient recycling and herbivory as mechanisms in the “top-down” effect of fish on algae in lakes. Ecol 78:21-40
Vanni MJ, Layne CD, Arnott SE (1997) “Top-down” trophic interactions in lakes: Effects of fish on nutrient dynamics. Ecol 78:1-20
Viana DS, Santamaria L, et al. (2014) Environment and biogeography drive aquatic plant and cladoceran species richness across Europe. Freshw Biol 59:2096-2106
Wetzel, R. G. (1983) Limnology (Saunders, Philadelphia), 2nd Ed.
Xie P, Yang Y (2000) Long-term changes of Copepoda community (1957–1996) in a subtropical Chinese lake stocked densely with planktivorous filterfeeding silver and bighead carp. J Plankton Res 22:1757–1778
Yasuno M, Morishita M, Sugaya Y (1977) Zoobenthos and zooplankton at Takahamairi Bay in Lake Kasumigaura. Res Rep Natl Inst Environ Stud 1:94-107 (in Japanese)
Yasuno M, Morishita M, Hanazato T (1981) Standing crop of zooplankton at Takahamairi Bay in Lake Kasumigaura. Res Rep Natl Inst Environ Stud 22:149-158 (in Japanese)