Metadata
Title
The densities of bacteria, picophytoplankton, heterotrophic nanoflagellates and ciliates in Lake Kasumigaura (Japan) monitored monthly since 1996
AUTHORS
Noriko Takamura and Megumi Nakagawa
Center for Environmental Biology and Ecosystem Studies, National Institute for Environmental Studies
*Author for correspondence
Noriko Takamura
National Institute for Environmental Studies
Email: cebes.data@nies.go.jp
Telephone number: +81-298-50-2471, Fax number: +81-298-50-2577
Address: National Institute for Environmental Studies, Onogawa 16-2, Tsukuba 305-8506, Japan
ABSTRACT
This data paper describes the densities of the bacterioplankton, picocyanobacteria, eukaryotic picoplankton, heterotrophic nanoflagellates (HNFs) and ciliates in the water of Lake Kasumigaura, a shallow, eutrophic lake that is the second largest in Japan. All of these planktonic organisms are components of a microbial loop and are countable using an epifluorescence microscope. These data represent the results of monthly collections from April 1996 through March 2010 at two sites on the lake, and this data set is unique among the available published data papers concerning lakes or plankton and continues to be freely available. The monitoring was performed as a component of the Lake Kasumigaura Long-term Environmental Monitoring program conducted by National Institute for Environmental Studies (NIES) since 1977 and includes water quality, plankton, and benthos. The data have been used for ecological studies and for studies on the management of water quality.
KEYWORDS
Lake Kasumigaura, picoplankton, bacteria, HNFs, ciliates, seasonal change, microbial food chain
INTRODUCTION
Micro-sized plankton, such as bacteria, picophytoplankton, heterotrophic nanoflagellates (HNFs), and ciliates play important functional roles in the cycling of matter in aquatic ecosystems (Hagstrom et al. 1988; Jumars et al. 1989; Sherr and Sherr 1994; Calbet and Landry 1999), and their roles became known in the 1980’s through the widespread use of epifluorescence microscopy. These organisms are recognized as the primary components of the microbial food web (Bennett et al. 1990; Sherr and Sherr 1991; Vaqué et al. 1997), yet their biomass in the aquatic environment is usually small compared with other plankton or nekton because they are actively incorporated into the food chain in the water. Nevertheless, their density is indicative of the trophic status of the waters that they inhabit (Takamura et al. 1996).
In Lake Biwa, the largest lake in Japan, the resident picocyanobacteria bloomed and reached an abundance greater than 106 cells mL-1 in the summers of 1989 and 1990 (Maeda et al. 1992). After the bloom, substantial amount of ayu fish (Plecoglossus altivelis altivelis) died although the direct causal relationship between the bloom and the death of ayu fish is still unclear. For this reason, data for these micro-sized plankton in aquatic ecosystems have been used in ecological studies and in studies on the management of lake environments (Christoffersen et al. 1990; Ventela et al. 2002).
The sustainability of our society depends on the ways in which we conserve various natural resources, including lakes. Plankton is one of the most important components in a lake ecosystem, and its turnover time is short, typically within several hours to days. Therefore, monthly monitoring over a long time period is necessary to evaluate the anthropogenic effects on lake ecosystems, predict the environment of these ecosystems and conserve them properly based on scientific knowledge.
This data paper reports the densities of the bacterioplankton, picocyanobacteria, eukaryotic picoplankton, HNFs, and ciliates in the water of Lake Kasumigaura, a shallow, eutrophic lake that is the second largest in Japan. All of these plankton are components of the microbial food web and are countable using an epifluorescence microscope. The reported data were collected monthly from April 1996 through March 2010 at two sites on the lake. This data set is unique among the published data papers concerning lakes or plankton and continues to be freely available.
The monitoring was performed as a component of the Lake Kasumigaura Long-term Environmental Monitoring program conducted by NIES since 1977. The program includes the collection of data on the water quality, plankton, and benthos, and the data set of this data paper is part of the database of the Lake Kasumigaura Long-term Environmental Monitoring program. Lake Kasumigaura is registered as a core site of the Japan Long-term Ecological Research Network (JaLTER), a member of the International Long-term Ecological Research Network (ILTER).
METADATA
1. Title
The densities of bacteria, picophytoplankton, heterotrophic nanoflagellates and ciliates in Lake Kasumigaura (Japan) monitored monthly since 1996
2. Identifier
ERDP-2012-03
3. Contributors
A. Principal investigators
Tomiji Hagiwara, Akio Imai, Kazuho Inaba, Kazuhiro Iwasaki, Nobuyuki Kawasaki, Ayato Kohzu, Kazuhiro Komatsu, Masami Koshikawa, Kazuo Matsushige, Shin-ichiro Matsuzaki, Megumi Nakagawa, Yukihiro Nojiri, Takayuki Satou, Noriko Tomioka, Ryuhei Ueno, Junko Yamamura, and Noriko Takamura conducted the field survey.
B. Data Set Owners
The National Institute for Environmental Studies, Japan, and the following individuals are the owners of the data.
| Owners and contact individual(*) | Affiliation | Contact | |||
|---|---|---|---|---|---|
| Address | Phone | Fax | Email address | ||
| Noriko Takamura*, Megumi Nakagawa | National Institute for Environmental Studies | Onogawa 16-2, Tsukuba 305-8506, Japan | +81-298-50-2471 | +81-298-50-2577 | noriko-t@nies.go.jp |
C. Data Set Creators
Noriko Takamura, Center for Environmental Biology and Ecosystem Studies, National Institute for Environmental Studies
Megumi Nakagawa, Center for Environmental Biology and Ecosystem Studies, National Institute for Environmental Studies
4. Program
A. Title
Lake Kasumigaura Long-term Environmental Monitoring program
B. Personal
Organization: National Institute for Environmental Studies
Address: Onogawa 16-2, Tsukuba 305-8506, Japan
Phone: +81-298-50-2471 (Voice)
Phone: +81-298-50-2577 (fax)
Web Address: http://www.nies.go.jp/
C. Funding
National Institute for Environmental Studies (NIES), Japan
D. Objectives
The National Institute for Environmental Studies (NIES) has conducted monthly monitoring of the water quality and plankton/benthos biomass in Lake Kasumigaura since 1977. The bacterioplankton, picocyanobacteria, eukaryotic picoplankton, HNFs, and ciliates, all of which are the components of a microbial food web in the water, have also been monitored since April 1996 to advance the ecological understanding of the ecosystem of the lake.
5. Geographic coverage
A. Geographic Description
Nishi-ura of Lake Kasumigaura, Japan
B. Geographical Position
Station 3: 36° 07.302′ N, 140° 22.652′ E (WGS84)
Station 9: 36° 02.142′ N, 140° 24.222′ E (WGS84)
6. Temporal coverage
A. Begin: April 1996
B. End: September 2015
7. Methods
A. Study sites
Lake Kasumigaura (Figure 1) is located approximately 60 km northeast of the Tokyo metropolitan area. It is the second largest lake in Japan (surface area of 220 km2, a total volume of 0.85 billion m3) and is shallow (mean depth of 4 m, maximum depth of 7 m), with a catchment area of 2157 km2. The lake is composed of three parts: Nishi-ura, Kita-ura and Sotonasaka-ura. Nishi-ura, the largest part of the lake, has a surface area of 167.7 km2 and a total volume of 662 million m3. This part of the lake has 29 inflows and 1 major outflow. The Lake Kasumigaura Long-term Environmental Monitoring program is conducted at 10 sites in Nishi-ura, and the densities of bacteria, picophytoplankton, HNFs, and ciliates are monitored at 2 sites. The annual means of the transparency and the concentrations of the total phosphorus (TP), total nitrogen (TN), chlorophyll a (Chl.a), and suspended solids (SS) at the two sites from 1996 and 2009 are shown in Figure 2. The history of the lake’s alteration by recent human activities and the associated environmental problems are summarized in Takamura (2012).
Fig.1. Sampling sites in Lake Kasumigaura (Nishi-Ura).
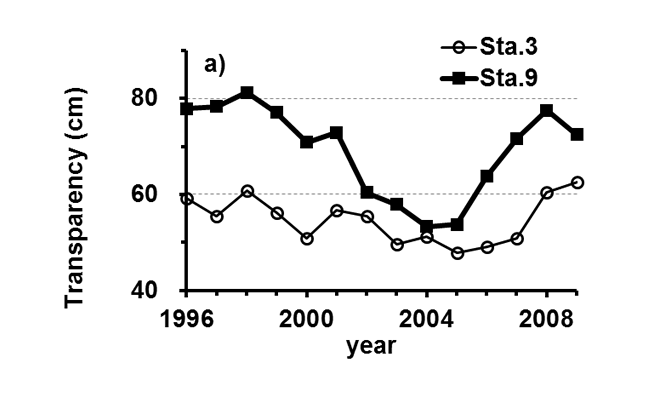
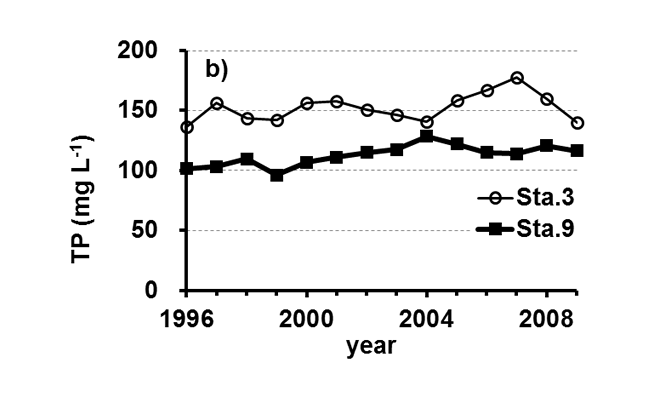
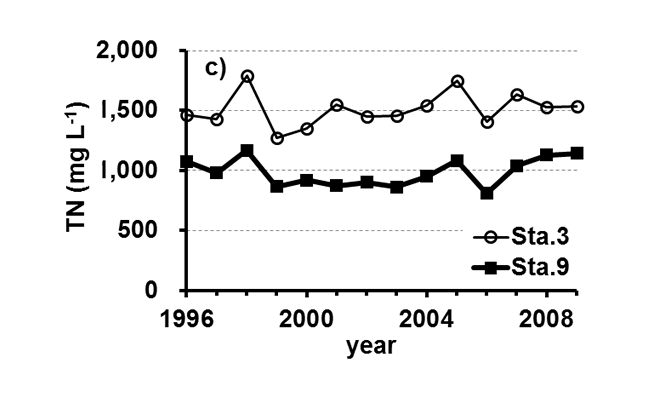
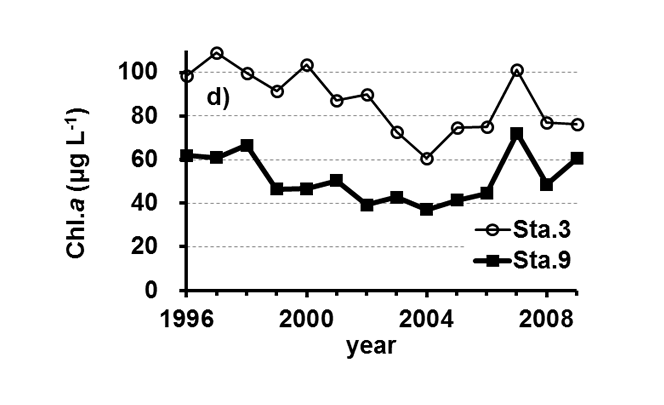
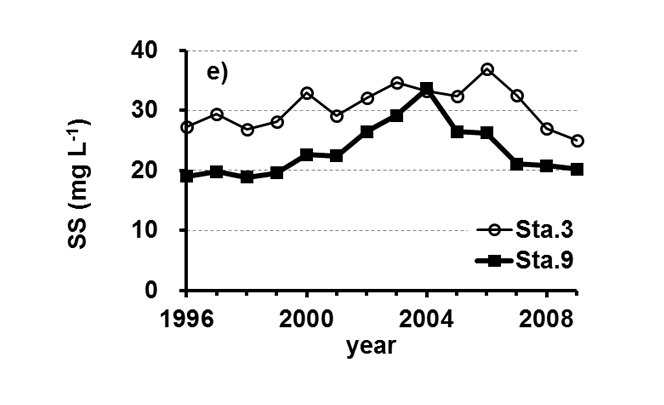
Fig.2. Changes in the annul means of the a) transparency, b) concentrations of the total phosphorus (TP), c) total nitrogen (TN), d) chlorophyll a (Chl.a), and e) suspended solids (SS) at Sta.3 and Sta.9.
B. Sampling and sample preservation methods
The water samples were collected using a column sampler from the surface to a depth of 2.0 m. The samples were immediately fixed with glutaraldehyde (final concentration of 1 %, v/v) for the enumeration of the bacteria, HNFs, and picophytoplankton and were fixed with Lugol's iodine solution for the counting of the ciliates. The samples were stored under dark and cool (approximately 4 ℃) conditions prior to counting in the laboratory.
C. Counting methods
The bacteria, picophytoplankton, HNFs, and ciliates were counted following Takamura et al. (1996). The bacteria, picophytoplankton (both picocyanobacteria and eukaryotic picoplankton), and HNFs were counted using an epifluorescence microscope within one week of the collection of the sample, and the ciliates were counted using an inverted microscope within 6 months.
To count the bacteria, 1-2 mL of the sample was diluted by 10 % with particle-free deionized water, and 1-2 mL of the diluted sample was then filtered through a 0.1-µm pore-size Nuclepore filter (stained with Sudan Black B; 25 mm in diameter). Immediately after filtration, a few drops of DAPI* solution were added to cover the entire surface of the filter. After 2-3 minutes, the drops were filtered to remove the excess dye.
To count the picocyanobacteria and eukaryotic picoplankton, 1-3 mL of the sample was mixed with particle-free deionized water. This diluted sample was then filtered through a 0.2-µm pore-size Nuclepore filter (stained with Sudan Black B; 25 mm in diameter).
To count the HNFs, 3-10 mL of the sample was filtered through a 1.0-µm pore-size Nuclepore filter (stained with Sudan Black B; 25 mm in diameter). After the filtration, a few drops of FITC** solution were added to cover the entire surface of the filter. After 2-3 minutes, the drops were filtered to remove the excess dye. Phosphate buffer was then added and the solution was filtered to rinse the filter.
All of the filtration steps were performed under a moderate vacuum.
The filters were placed on slide glasses and mounted in low-fluorescence immersion oil. The filter was observed in a darkened room, and the bacteria were counted using an epifluorescence microscope (Olympus BX51, Tokyo) equipped with a U-excitation system (Olympus BX-RFA, Tokyo). The picocyanobacteria were observed using an epifluorescence microscope equipped with a G-excitation system. Both the eukaryotic picoplankton and HNFs were observed using a BV-excitation system.
The objective particles were counted in frames defined eyepiece grids. The grid was selected randomly. The bacterial cells and picocyanobacterial cells were counted until the total number exceeded 400 cells, whereas the eukaryotic picoplankton and HNFs were counted until the total number exceeded 100 cells.
The ciliates were counted using an inverted microscope after the plankton in 3-10 mL of sample settled for 24 hours in a Utermöhl chamber (1958). The entire area or half of the area of the chamber was counted.
*DAPI (4'6-diamidino-2-phenylindole) solution: 100 µg of DAPI was added to 10 mL of S-buffer (0.25 M sucrose, 1 mM EDTA, 0.6 mM spermidine, 0.05 % 2-mercaptoethanol and 10 mM Tris-HCL adjusted to pH 7.6).
**FITC (fluorescein isothiocyanate) solution: 2 mg of FITC was added to 50 mL of phosphate buffer (1.3 g NaH2PO4·2H2O and 8.6 g NaHPO4·12H2O with the volume was adjusted to 500 mL with deionized water and the pH adjusted to 7.2); the solution was filtered through a 0.45-µm membrane filter.
D. Data verification procedures
The numbers representing the count data include errors of ± 10 % for the bacteria and picocyanobacteria and ± 20 % for the eukaryotic picoplankton, HNFs and ciliates. The precision did not change during the 1996–2015 monitoring. The data were manually digitized and checked for typographical errors by the investigators. If any suspicious value remained, it was recorded as an error (see section 10.D).
8. Data Status
A. Latest Update
11 September 2015
The reported data were collected from April 1996 through September 2015. The collection of the data has continued since September 2015. The database will be updated as these data are verified.
B. Metadata Status
The metadata are complete for this period and are stored with the data.
9. Accessibility
A. License and Usage Rights
1) Acceptable use. The data set should not be used for illegal purposes or to violate the rights of others. The use of the data set will be restricted to academic, research, educational, governmental, or other not-for-profit professional purposes. Data users need to agree to the terms of use for NIES Lake Kasumigaura Database (http://db.cger.nies.go.jp/gem/moni-e/inter/GEMS/database/kasumi/contents/terms.html). Please make sure to contact the data manager of Lake Kasumigaura Database by email (cebes.data@nies.go.jp) before using the dataset.
2) Citation. Data users should properly cite this data paper in any publications or in the metadata of any derived data products that were produced using the data set. Because the data set will be updated and the measuring methods will be modified as the techniques continue to develop, please check the latest version of the data set.
3) Acknowledgement. To support this long-term monitoring activity, data users should write acknowledgements in any publications to whose content the data set contributed as follows: “Data for XXX were provided by Lake Kasumigaura Long-term Environmental Monitoring program of the National Institute for Environmental Studies, Japan”.
4) Notification. Data users should notify the Data Set Contact when any derivative work or publication based on or derived from the Data Set is distributed. Inform the Data Set Contact with two reprints or a PDF file of any publications resulting from the use of the data set.
5) Collaboration. Data users are strongly encouraged to consider consultation, collaboration and/or co-authorship with the data owners.
6) Disclaimer. In no event shall the authors, data owners, or the National Institute for Environmental Studies be liable for a loss of profits, or for any indirect, incidental damages arising from the use or interpretation of the data.
B. Contact
Data Set Contact
Noriko Takamura or Megumi Nakagawa
Center for Environmental Biology and Ecosystem Studies, the National Institute for Environmental Studies, Onogawa 16-2, Tsukuba 305-8506, Japan
Telephone number: +81-298-50-2471
Email: cebes.data@nies.go.jp
C. Storage location
http://db.cger.nies.go.jp/JaLTER/metacat/metacat/ERDP-2012-03.1/default
The data owners and the National Institute for Environmental Studies store the original data.
10. Data Structure
A. Data table
| Data file name | Description |
|---|---|
| Kasumigaura_Microbialplankton.csv | Densities (cells mL-1) of bacteria, HNFs, ciliates, picocyanobacteria and eukaryotic picoplankton in the water. |
B. Format type
The data file is in ASCII text, comma delimited (csv).
C. Header information
Headers corresponding to variable names (see section 10.D) are included as the first row in the data file.
D. Variable definitions
The variables are listed in the order they appear in the data file. The variable names are headers included as the first row in the data file. "NA" is the code for errors and missing values for all of the variables.
| Data file name | Variable name | Variable definition | Range of numerical values |
|---|---|---|---|
| Kasumigaura_Microbialplankton.csv | Date | Sampling date (yyyy/m/d) | 1996/4/3 - 2015/9/11 |
| Station (Sta.) | Site name | 3, 9 | |
| Bacteria | Cell number of bacteria in the water (cells mL-1) | 1150000 - 94980555 | |
| HNFs | Cell number of heterotrophic nanoflagellates in the water (cells mL-1) | 50 - 27584 | |
| Ciliates | Cell number of ciliates in the water (cells mL-1) | 3 - 727 | |
| Picocyanobacteria | Cell number of picocyanobacteria in the water (cells mL-1) | 38 - 2582690 | |
| Eukaryotic picoplankton | Cell number of eukaryotic picoplankton in the water (cells mL-1) | 0 - 89455 |
11. Supplementary information
The data set of this data paper represents part of the database of the Lake Kasumigaura Long-term Environmental Monitoring program. This monitoring program has been conducted by NIES since 1977, and the data in this data paper are therefore, fully identical to the data reported on the Japanese ( http://db.cger.nies.go.jp/gem/inter/GEMS/database/kasumi/index.html ) and English ( http://db.cger.nies.go.jp/gem/moni-e/inter/GEMS/database/kasumi/index.html ) websites of NIES, Japan, although the data on the website cover limited time period of the data of this data paper.
The Lake Kasumigaura Long-term Environmental Monitoring program measures selected environmental variables (water temperature, water depth, transparency, dissolved oxygen, pH, and light intensity in the water), water quality (EC, COD, Chl.a, SS, POC, PON, TP, DTP, SRP, TN, DTN,NH4-N, NO2-N+NO3-N, Al, B, Ba, Ca, Fe, K, Mg, Mn, Na, Si, Sr, and Cu), plankton (bacteria, HNFs, ciliates, picocyanobacteria, eukaryotic picoplankton, phytoplankton, rotifer, crustacean zooplankton, and mysids), benthos (chironomids and oligochaetes), and primary production.
A list of publications associated with Lake Kasumigaura Long-term Environmental Monitoring program is shown on the website http://db.cger.nies.go.jp/gem/inter/GEMS/database/kasumi/contents/research.html .
12. Acknowledgements
The publication of this data paper was encouraged through the partial support by J-BON (Japan Biodiversity Observation Network) and the Environmental Research and Technology Development Fund (S9) of the Ministry of the Environment, Japan. We thank the two anonymous reviewers for valuable comments.
13. Literature cited
Bennett SJ, Sanders RW, Porter KG (1990) Heterotrophic, autotrophic, and mixotrophic nanoflagellates - seasonal abundances and bacterivory in a eutrophic lake. Limnology and Oceanography 35:1821-1832
Calbet A, Landry MR (1999) Mesozooplankton influences on the microbial food web: Direct and indirect trophic interactions in the oligotrophic open ocean. Limnology and Oceanography 44:1370-1380
Christoffersen K, Riemann B, Hansen LR, Klysner A, Sorensen HB (1990) Qualitative importance of the microbial loop and plankton community structure in a eutrophic lake during a bloom of cyanobacteria. Microbial Ecology 20:253-272
Hagstrom A, Azam F, Andersson A, Wikner J, Rassoulzadegan F (1988) Microbial loop in an oligotrophic pelagic marine ecosystem - possible roles of cyanobacteria and nanoflagellates in the organic fluxes. Marine Ecology-Progress Series 49:171-178
Jumars PA, Penry DL, Baross JA , Perry MJ, Frost BW (1989) Closing the microbial loop - dissolved carbon pathway to heterotrophic bacteria from incomplete ingestion, digestion and absorption in animals. Deep-Sea Research Part A- Oceanographic Research Papers 36:483-495
Maeda H, Kawai A, Tilzer MM (1992) The water bloom of Cyanobacterial picoplankton in Lake Biwa, Japan. Hydrobiologia 248:93-103
Sherr EB, Sherr BF (1991) Planktonic microbes – Tiny cells at the base of the oceans food webs. Trends in Ecology and Evolution 6:50-54
Sherr EB, Sherr BF (1994) Bacterivory and herbivory – key roles of phagotrophic protists in pelagic food webs. Microbial Ecology 28:223-235
Takamura N (2012) The status of biodiversity loss in lakes and ponds in Japan. In: Nakano S, Yahara T, Nakashizuka T (eds) Biodiversity Observation Network in Asia-Pacific region: Towards further development of monitoring activities, Springer, Tokyo
Takamura N, Ishikawa Y, Mikami H, Fujita Y, Higuchi S, Murase H, Yamanaka S, Nan-jyo Y, Igari T, Fukushima T (1996) Abundance of bacteria, picophytoplankton, nanoflagellates and ciliates in relation to chlorophyll a and nutrient concentrations in 34 Japanese waters. Japanese Journal of Limnology 57:245-259 (in Japanese with English summary)
Utermöhl H (1958) Zur Vervollkommung der quantitative phytoplankton-Methodik. Internationale Vereinigung für theoretishe und angewandte Limnologie, Mitteilungen 9:1-38
Vaqué D, Blough HA, Duarte CM (1997) Dynamics of ciliate abundance, biomass and community composition in an oligotrophic coastal environment (NW Mediterranean). Aquatic Microbial Ecology 12:71-83
Ventela AM, Wiackowski K, Moilanen M, Saarikari V, Vuorio K, Sarvala J (2002) The effect of small zooplankton on the microbial loop and edible algae during a cyanobacterial bloom. Freshwater Biology 47:1807-1819