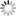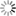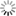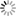Metadata
Title
Native Mediterranean plants as potential food sources for natural enemies of insect pests in olive groves
Authors
Anabela Nave*a, António L. Crespía, Fátima Gonçalvesa, Mercedes Camposb, Laura Torresa
- a Centre for the Research and Technology of Agro-Environmental and Biological Sciences, University of Trás-os-Montes and Alto Douro, Quinta de Prados, 5001-801, Vila Real, Portugal
- b Department of Environmental Protection, Experimental Station Zaidín, Profesor Albareda nº1, 18008 - Granada, Spain
*
Corresponding author: ana.nave@hotmail.com
Phone: +351 259 350 475
Fax: +351 259 350 629
Abstract
The diversity of native non-crop (“weed”) vegetation in agricultural landscapes can provide arthropod natural enemies with food sources and shelter, thus improving natural pest control and reducing dependence on chemical pesticides. Moreover, native plants to a region are uniquely positioned to provide cultural ecosystem services such as wild food and wild medicinal plants, as well as aesthetics values. The Mediterranean Basin is one of the world’s richest places in terms of plant diversity. Olive cultivation is the basic tree cultivation in the Mediterranean and dominates its rural landscape. The olive grove ecosystem, whose flora presents a notable resemblance to the flora of Mediterranean type ecosystems, is home to a myriad of species of insects, spiders and other arthropods. This includes over one hundred phytophagous species, plus an uncounted number of entomophagous that help to reduce phytophagous populations. Here we present data on flowering plant species from the ground cover of olive groves, store information on characteristics of plant species namely physiognomic type and flowering period, geographic information and some statistical values on olive groves study area and records in the flora of visitor arthropods and cultural ecosystem services. The data include information on 36 olive groves, 100 flora species (taxa), of which 86 native in Portugal, 5 endemic to Iberian Peninsula and 4 endemic to Portugal Continental, and present also a summary of the records of visitor arthropods in these flora (i.e. 2 classes, 6 orders and 12 families).
Keywords
- Aesthetic value
- Ecosystem services
- Endemic plants
- Nectar
- Parasitoid
- Pollen
- Predator
- Shelter
- Wild edible plants
- Wild medicinal plants
Introduction
The agricultural intensification, with associated reduction and fragmentation of non-crop habitats, intensive use of pesticides and high levels of disturbance, has greatly simplified farming landscapes, resulting in a sharp decline in on-farm biodiversity (Wade et al., 2008; Lu et al., 2014). A key aspect of this decline is the negative impact in the survival of beneficial arthropods and the supply of arthropod-mediated ecosystem services within crop fields. To provide these critical services beneficial arthropods depend on a variety of plant resources in their environment including shelter and overwintering sites, alternative hosts or prey and nectar and pollen (Landis et al., 2000; Jonsson et al., 2008; Lundgren, 2009). Understanding the value of native plants to a region in terms of the provision of ecosystem services, presents opportunities for their maintenance and/or reincorporation into agricultural landscapes (Landis et al., 2012). Services provided by these plants include besides enhancing natural pest control, conservation of biodiversity, soil and water conservation, and weed suppression. Moreover, in many cultures, native plants have important medicinal, religious and aesthetic values (Fiedler et al., 2008; Landis et al., 2012).
The Mediterranean Basin is one of the world’s richest places in terms of plant diversity. Thus, about 25,000 species are native to the region, and more than half of these (i.e. 13,000) are endemic, which means that they are not found anywhere else on earth (Cuttelod et al., 2008). The Portugal flora, includes a total of 3995 taxa (including infraspecific taxa), distributed among 1066 genera and 185 families, being referenced 135 endemic species (Menezes de Sequeira et al., 2012).
Several conditions, reviewed in Vogiatzakis et al. (2006), has proved particularly advantageous for the development of this high biodiversity. These are, namely: (i) the location of the basin that allowed floral and faunal elements to merge at the junction of three continents; (ii) the relative climatic uniformity throughout the region, with a summer drought and winter rainfall and (iii) the elevated temperatures during the summer drought and the accumulation of dry biomass which accelerates nutrient recycling.
Due to the above exposed, the Mediterranean Basin should be considered as a hyper-hot candidate for conservation support (Myers et al., 2000).
The olive tree (Olea europaea L. ssp. sativa Hoffman & Link) represents along with grapevine and cereal growing, the most traditional agricultural activity of the Mediterranean world and the most outstanding characteristic of their agricultural landscape (Angles, 1999). In addition to their unquestionable cultural and landscape values, traditionally managed olive groves have a major environmental importance since that often support a rich ground flora which may include species of considerable conservation interest (Perrino et al., 2014). Olive groves are, according to Allen (2009), one of the few examples of Mediterranean “semi-natural” landscapes, where some vegetation communities could develop alongside cultivation practices. Typically, the ground flora includes annuals and geophytes (bulbs) species. The annuals survive because regular shallow tilling and disturbance of the soil promotes germination of seeds; the geophytes survive because they are buried beneath the depth of tillage (Allen, 2009).
In support of the above it is noted that, as stated by Loumou and Giourga (2003), one of the principal types of Mediterranean ecosystems is, according to phytogeographers, the Oleo-Ceratonion, which, as its name suggests, is characterized by the presence of olive tree. Additionally, it is believed that through the process of ecological succession, the abandoned olive groves tend to turn into natural forests of the Mediterranean type, depending on the climatic and territorial conditions of each region (reference in Loumou and Giourga, 2003).
The occurrence, in the ground flora of traditionally managed olive groves, of a significant number of diverse plants of the Mediterranean flora (references in Loumou and Giourga, 2003) promotes the conditions for the existence of a lot of habitats for invertebrates. As a result it has a wealth of arthropod fauna that in addition to one hundred or so phytophagous species includes a large complex of entomophagous that help to reduce pest numbers (Viggiani, 1986). Although the most numerous entomophagous of this fauna are Hymenoptera parasitoids, of which a large number of species have now been recorded (e.g. Villa et al., 2016; Nave et al., in press), other groups are common, such as Tachinidae (Tschorsnig et al., 2011) also in the parasitoids, and Araneae, Anthocoridae, Chrysopidae, Coccinellidae, Formicidae and Syrphidae (e.g. Santos et al., 2009; Pascual et al., 2010; Paredes et al., 2013; Pinheiro et al., 2013a; b; 2015; Gonzalez et al., 2016), in the predators.
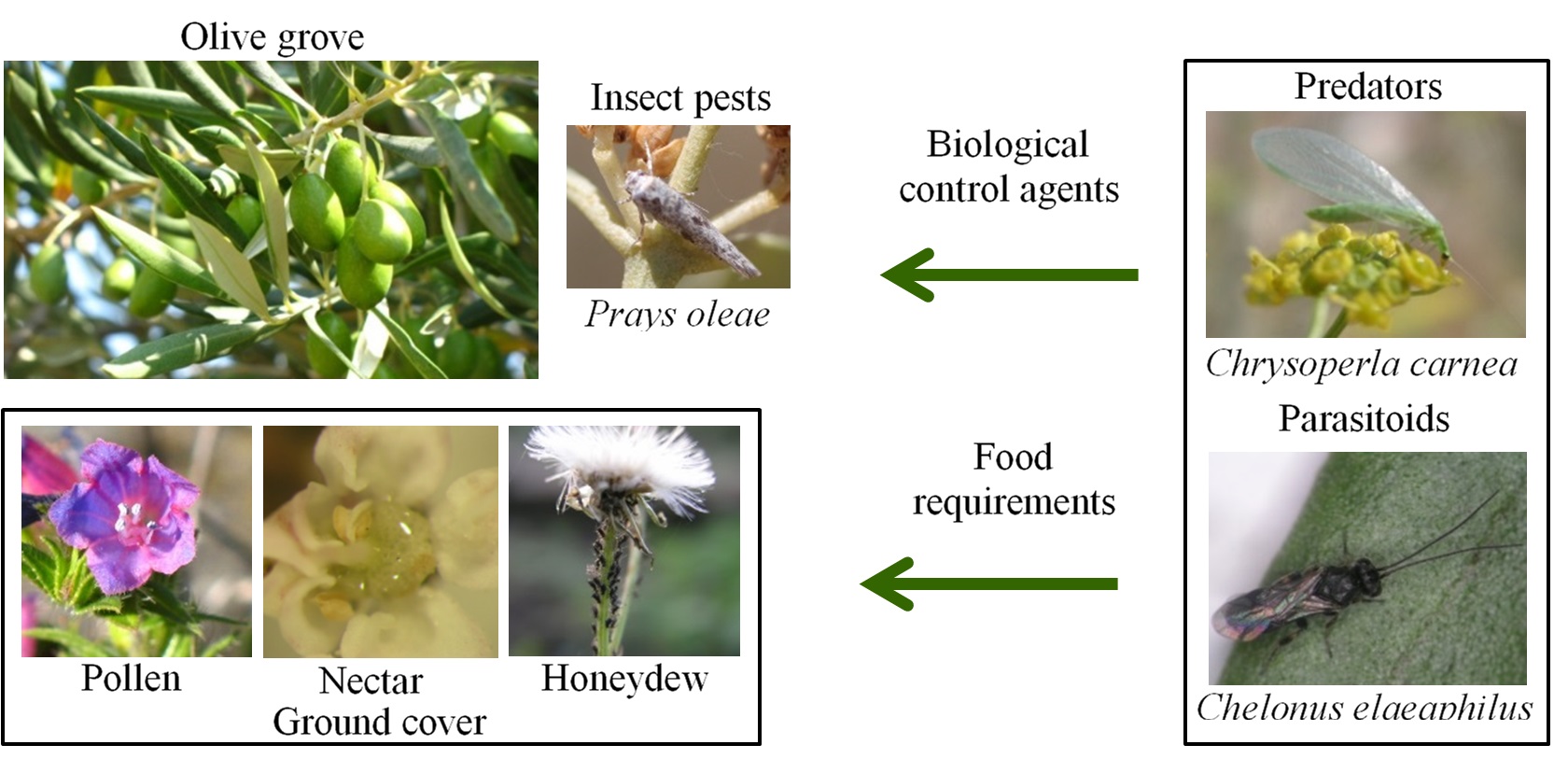
However and although the potential role of non-crop (“weed”) vegetation between olive trees in the conservation and augmentation of natural enemies of olive pests has long been recognized (e.g. Jervis et al., 1992), to date few data have been published identifying the native Mediterranean plant species that could potentially provide food and/or shelter resources to these beneficials (Fig. 1).
This study aimed to: a) identify the species of spontaneous flora present in the natural ground cover of traditional olive groves in Portugal; b) characterize the identified species in terms of family, physiognomic type and flowering period; c) summarize, on the basis of the available literature, the records of visitor arthropods in the identified plant species (in order to evaluate their potential in improving pest control services), as well as their interest from the standpoint of either ethnobotanical uses or aesthetic value (i.e. provision of cultural ecosystem services).
The provided information could be useful in promoting the maintenance and/or reincorporation of the studied plants into the olive grove ecosystem or even into other Mediterranean agroecosystems. So, while the role of these plants in improving pest management may be attractive to growers, the provision of cultural ecosystem services such as the wild food, wild medicines and aesthetic values, may make it far more likely that society at large will support their implementation.
GEOGRAPHIC COVERAGE
Portugal - districts of Castelo Branco (39°48' N and 7°30' W),
Guarda (40°32' N and 7°15' W), and Viseu (40°39' N and 7°54' W).
TEMPORAL COVERAGE
October and November of 2008
TAXONOMIC COVERAGE
Flora
The data include 80 genera and 100 species (see FPS.txt for detail).
Arthropods
Summary of the records of visitor arthropods in the flora based on references in available literature. The data include 2 classes, 6 orders and 12 families (see PSIF.txt for detail).
METHODS
Study area
Floristic inventories were done in 36 olive groves, with the soil covered with natural vegetation, in order to identify the plant species presents. The distribution of the olive groves by district (Fig. 2) was as follows: a) Guarda, 29 groves distributed by counties of Mêda, Guarda, Gouveia, Seia, Trancoso, Figueira de Castelo Rodrigo, Celorico da Beira and Pinhel, b) Castelo Branco, five groves distributed by counties of Belmonte, Covilhã and Fundão and c) Viseu, two groves, located in the county of Penedono (see OGSA.txt for detail). The olive groves were traditional plantations, rain-fed, managed with few or no chemical inputs, but with a high labour input. They have different ages (young to more than 60 years), plant spacing variable (from 5 × 3 m, to 8 × 8 m), including also scattered trees, from olive tree monovarietal to olive groves with several varieties (i.e. Galega, Cornicabra, Cobrançosa, Picual, Negrinha , Madural and Cordovil).
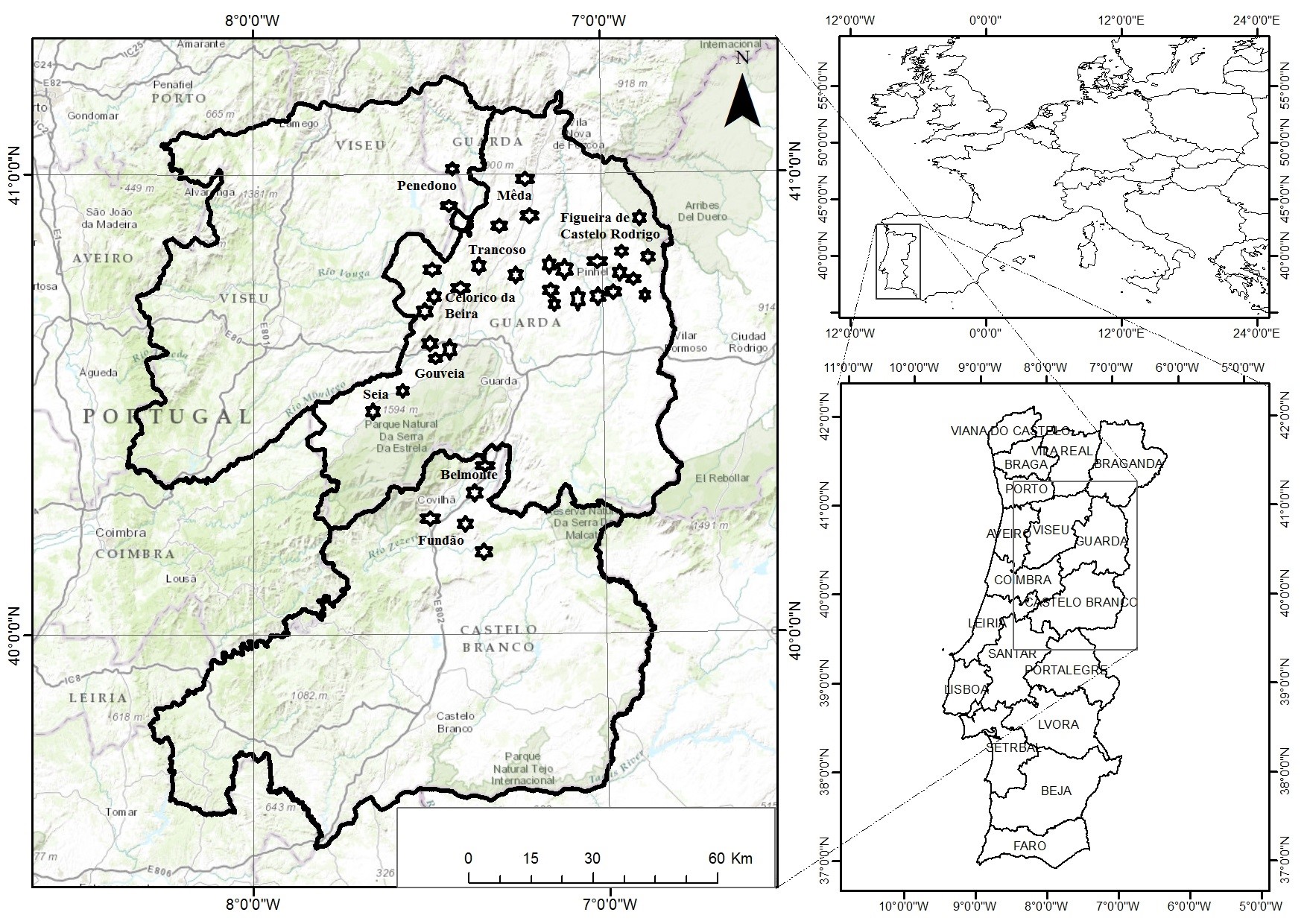 Fig 2 – Map of study area showing the
distribution of the groves where floristic inventories were done.
Fig 2 – Map of study area showing the
distribution of the groves where floristic inventories were done.
Sampling methods
For the inventories, in each of the olive grove, 10 sampling units with 1 x 1m of surface were chosen according to chance (i.e. random sampling) but as representative of vegetation, dispersed throughout the entire survey area in a flexible sampling scheme. We made one flowering plants collection in each olive grove and all the plants were subject to confirmation its identification in the laboratory, based on the keys of Iberian plants (Castroviejo 1997). For each species the flowering period was recorded (Castroviejo 1997), life form was classified on the basis of the system of physiognomic types proposed by Raunkiaer (1934) and the natural occurrence and endemism registered from Flora-on (2016) (see PSC.txt for detail).
COLLECTOR DATA
Name: Anabela Nave
Affiliation:
Centre for the Research and Technology of Agro-Environmental and Biological Sciences,
University of Trás-os-Montes and Alto Douro
Address:
Quinta de Prados, 5001-801, Vila Real, Portugal
Mail: ana.nave@hotmail.com
EXPERT SPECIES IDENTIFICATION
Name: António Luís Crespi
Affiliation:
Centre for the Research and Technology of Agro-Environmental and Biological Sciences,
University of Trás-os-Montes and Alto Douro
Address:
Quinta de Prados, 5001-801, Vila Real, Portugal
Mail: acrespi@utad.pt
DATA FORMAT
The published data were provided in a full dataset of records.
Dataset components
| Data file name | Description |
|---|---|
| FPS.txt | Flowering plant species from the ground cover of the studied olive groves. |
| PSC.txt | Store information on characteristics of plant species. |
| OGSA.txt | Geographic information and some statistical values on olive groves study area. |
| PSIF.txt | Records in the flora of visitor arthropods |
Format type
The data tables are prepared as comma delimited text files.
Data table descriptions
| Contents name | Description |
|---|---|
| Scientific name of plant species | The scientific name of plant species. |
| Specimens present in olive grove | Name of grove and number of specimens present. |
| Contents name | Description |
|---|---|
| Scientific name of plant species | The scientific name of each plant species. |
| Family | Family of each plant species. |
| Physiognomic type | Therophyte, Hemicryptophyte, Microphanerophyte, Nanophanerophyte, Geophyte and Chamaephyte. |
| Flowering period | Months of records. |
| Endemic to | Iberian Peninsula, Portugal Continental, No. |
| Natural in Portugal | Yes, No. |
| Cultural ecosystem services (references) | 0 = without reference, 1 = wild medicinal, 2 = wild edible, 3 = ornamental (a - Shrewsbury et al. (2004), b - Tardio et al. (2006), c - Rivera et al. (2006), d - Hadjichambis et al. (2008), e - Carvalho and Morales (2010), f - Parada et al. (2011), g - Carlos et al. (2012), h - Łuczaj et al. (2012), i – Molina et al. (2014), j – Gonzalez et al. (2016)). |
| Contents name | Description |
|---|---|
| Olive grove | Name of olive grove. |
| Olive grove district | District name. |
| District populations | Habitants per district. |
| Olive grove county | County name. |
| County populations | Habitants per county. |
| County usable agricultural area | Hectares of agricultural area. |
| Olive grove locality | Locality name. |
| Locality population density | (Hab./km²) (Eurostat, 2011). |
| Degree of urbanization | Densely, intermediate or thinly populated areas (Eurostat, 2011). |
| Decimal latitude | Olive grove decimal latitude. |
| Decimal longitude | Olive grove decimal longitude. |
| Contents name | Description |
|---|---|
| Scientific name of plant species | The scientific name of plant species. |
| Nectar | 0 = without reference, N = nectar (rich in amino acids and sugar). |
| Pollen | 0 = without reference, P = pollen (rich in amino acids and proteins). |
| Class of visitor | Insecta, Arachnida. |
| Order of visitor | Araneae, Coleoptera, Diptera, Hemiptera, Hymenoptera, Neuroptera. |
| Family of visitor | Anthocoridae, Apidae, Apoidea, Braconidae, Cecidomyiidae, Chalcidoidea, Chrysopidae, Coccinellidae, Formicidae, Ichneumonidae, Syrphidae, Tachinidae, 0 = without reference. |
| Genus of visitor | Apis, Chrysoperla, Orius, 0 = without reference. |
| Hibernation site | Hibernation site, 0 = without reference. |
| Ample prey and host animals | Ample prey and host animals, 0 = without reference. |
| Study for Conservation Biological Control (CBC) | Study for CBC, 0 = without reference. |
| References | 1 - Maingay et al. (1991), 2 - Freeman-Long et al. (1998), 3 - Colley and Luna (2000), 4 - Denys and Tscharntke (2002), 5 - English-Loeb et al. (2003), 6 – Böller et al. (2004), 7 - Fitzgerald and Solomon (2004), 8 - Rogers and Potter (2004), 9 - Wäckers (2004), 10 - Petanidou (2005), 11 - Villenave et al. (2005), 12 - Hanks et al. (2005), 13 - Rebek et al. (2005), 14 – Forehand et al. (2006), 15 - Pontin et al. (2006), 16 - Winkler et al. (2009), 17 – Ferreira et al. (2009), 18 – Tompkins et al. (2010), 19 - Saeed and Sajjad (2010), 20 - Sivinski et al. (2011), 21 - Al-Dobai et al. (2012), 22 - Carlos et al. (2012), 23 - Pinheiro et al. (2013a), 24 - Pinheiro et al. (2013b), 25 – Gonzalez et al. (2016). |
ACCESSIBILITY
License and Usage Rights
This dataset is provided under a Creative Commons Attribution 4.0 International license (CC-BY 4.0) (https://creativecommons.org/licenses/by/4.0/legalcode).
All published data can be used freely with reference to the data paper. In no event shall the authors or the data set owners be liable for any loss of profits or for any indirect, incidental, or consequential damages arising from the use of the data set.
ACKNOWLEDGMENTS
This study was supported by FEDER Funds throughout Programa Operacional Factores de Competitividade - COMPETE and National Funds throughout FCT - Fundação para a Ciência e Tecnologia, within project PTDC/AGR-AAM/100979/2008 – “Increasing functional biodiversity in olive groves to enhance conservation biological control of insect pests”, as well as PhD grant, SFRH/BD/34394/2008 attributed to the first author. This manuscript is part of A. Nave´s Ph.D. dissertation. The authors are grateful to the olive grove owners who permitted access to the groves for this study, with support to Associação de Agricultores para Produção Integrada de Frutos de Montanha.
REFERENCES
Al-Dobai S, Reitz S, Sivinski J (2012) Tachinidae (Diptera) associated with flowering plants: estimating floral attractiveness. Biol Control 61: 230–239
Allen HD (2009) Vegetation and Ecosystem Dynamics. In: Woodward JC. (ed) The Physical Geography of the Mediterranean, Oxford University Press Inc. New York, pp 203–227.
Angles S (1999) The changes in the olive-growing geography of Andalusia. Olivae 78: 12–22.
Böller EF, Häni F, Poehling H-M (2004) Ecological infrastructures: Ideabook on functional biodiversity at the farm level. Temperate zones of Europe. Swiss Centre for Agricultural Extension and Rural Development, Switzerland.
Carlos C, Sousa S, Nave A, Gonçalves F, Fernandes R, Crespí A, Torres L (2012) Artrópodos associados à flora da Região Demarcada do Douro. Workshop “Valorização de Serviços do Ecossistema na Atividade Vitivinícola”. Tabuaço, 28 Maio. Advid. http://www.advid.pt/imagens/workshops/poster%20ADVID_24_05.pdf
Carvalho AM, Morales R (2010) Persistence of wild food and wild medicinal plant knowledge in a northeastern region of Portugal. In: Pardo-de-Santayana M, Pieroni A, Puri RK (eds) Ethnobotany in the new Europe: people, health, and wild plant resources. New York NY: Berghahn Books, pp 147–171.
Castroviejo S (1997) Flora iberica: plantas vasculares de la Península Ibérica e Islas Baleares Santiago Castroviejo, Real Jardín Botánico, Spain.
Colley M, Luna J (2000) Relative Attractiveness of Potential Beneficial Insectary Plants to Aphidophagous Hoverflies (Diptera: Syrphidae). Environ Entomol 29: 1054-1059.
Cuttelod A, García N, Malak D, Temple H, Katariya V (2008) The Mediterranean: a biodiversity hotspot under threat. In: Vié J-C, Hilton-Taylor C, Stuart SN (eds) The 2008 Review of The IUCN Red List of Threatened Species. IUCN Gland, Switzerland.
Denys C, Tscharntke T (2002) Plant-insect communities and predator prey ratios in field margin strips, adjacent crop fields, and fallows. Oecologia 130: 315–324.
English-Loeb G, Rhainds M, Martinson T, Ugine T (2003) Influence of flowering cover crops on Anagrus parasitoids (Hymenoptera: Mymaridae) and Erythroneura leafhoppers (Homoptera: Cicadellidae) in New York vineyards. Agr Forest Entomol 5: 173–181.
Ferreira J, Torres L, Franco JC (2009) Limitação natural de pragas – valorização da actividade dos auxiliares. As bases da agricultura Biológica – Tomo I Produção Vegetal. (eds Edibio) pp. 370-382. Castelo de Paiva.
Fiedler AK, Landis DA, Wratten SD (2008) Maximizing ecosystem services from conservation biological control: the role of habitat management. Biol Control 45: 254-271.
Fitzgerald JD, Solomon MG (2004) Can flowering plants enhance numbers of beneficial arthropods in UK apple and pear orchards? Biocontrol Sci Techn 14: 291–300.
Flora-On (2016) Flora de Portugal interactiva. Sociedade Portuguesa de Botânica. http://www.flora-on.pt/#1endemismos. Accessed 14 December 2016.
Forehand LM, Orr DB, Linker HM (2006) Evaluation of a commercially available beneficial insect habitat for management of Lepidoptera pests. J Econ Entomol 99: 641–647.
Freeman-Long R, Corbett A, Lamb C, Reberg-Horton C, Chandler J, Stimmann M (1998) Beneficial insects move from flowering plants to nearby crops. Calif Agric 52: 23–26.
Gonzalez D, Nave A, Gonçalves F, Nunes FM, Campos M, Torres L (2016) Higher longevity and fecundity of Chrysoperla carnea, a predator of olive pests, on some native flowering Mediterranean plants. Agron Sustain Dev 36: 1-10.
Hadjichambis A, Paraskeva-Hadjichambi D, Della A, Elena Giusti M, De Pasquale C, Lenzarini C, Censorii E, Gonzales-Tejero M, Sanchez-Rojas C, Ramiro-Gutierrez J, Skoula M, Johnson C, Sarpaki A, Hmamouchi M, Jorhi S, EL-Demerdash M, EL-Zayat M, Pieroni A (2008) Wild and semi-domesticated food plant consumption in seven circum-Mediterranean areas. Int J Food Sci Nutr 59: 383–414.
Hanks L, Sadof C, Rebek E (2005) Manipulating the Abundance of Natural Enemies in Ornamental Landscapes with Floral Resource Plants. Biol Control 33: 203-216.
Jervis MA, Kidd NAC, McEwen P, Campos M, Lozano C (1992) Biological control strategies in olive pest management. Research Collaboration in European IPM Systems. BCPC Monograph nº 52. In: Haskell PT (ed.) Proceedings of a special Introductory Session held at the Brighton Crop Protection Conference – Pests and Diseases, pp 31-39.
Jonsson M, Wratten SD, Landis DA Gurr, GM (2008) Recent advances in conservation biological control of arthropods by arthropods. Biol Control 45: 172–175.
Landis D, Wratten S, Gurr G (2000) Habitat management to conserve natural enemies of arthropod pests in agriculture. Annu Rev Entomol 45: 175–201.
Landis DA, Gardiner MM and Tompkins J (2012) Using native plant species to diversify agriculture. In: Geoff MG, Wratten SD, Snyder WE (eds) Biodiversity and insect pests: key issues for sustainable management. Wiley-Blackwell, Oxford, pp 276–292.
Loumou A, Giourga C (2003) Olive groves: “The life and identity of the Mediterranean”. Agric Human Values 20: 87–95.
Lu ZX, Zhu PY, Gurr GM, Zheng XS, Read DM, Heong KL, Yang YJ, Xu HX (2014) Mechanisms for flowering plants to benefit arthropod natural enemies of insect pests: prospects for enhanced use in agriculture. Insect Sci 21: 1–12.
Łuczaj Ł, Pieroni A, Tardío J, Pardo-de-Santayana M, Sõukand R, Svanberg I, Kalle R (2012) Wild food plant use in 21st century Europe: the disappearance of old traditions and the search for new cuisines involving wild edibles. Acta Soc Bot Pol 81: 359-370.
Lundgren JG (2009) Relationships of Natural Enemies and Non-prey Foods. Progress in Biological Control. Springer, 453 pp.
Myers N, Mittermeier RA, Mittermeier CG, da Fonseca GAB, Kent J (2000) Biodiversity hotspots for conservation priorities. Nature 403: 853–858.
Menezes de Sequeira M, Espírito-Santo D, Aguiar C, Capelo J, Honrado J (2012) Checklist da Flora de Portugal (Continental, Açores e Madeira). Associação Lusitana de Fitossociologia. Lisboa, 74 pp. ISBN: 978-989-20-2690-9.
Maingay HM, Bugg RL, Carlson RW, Davidson NA (1991) Predatory and parasitic wasps (Hymenoptera) feeding at flowers of sweet fennel (Foeniculum vulgare Miller Var Dulce Battandier and Trabut, Apiaceae) and spearmint (Mentha spicata L, Lamiaceae) in Massachusetts. Biol Agric Hortic 7: 363–383.
Molina M, Tardío J, Aceituno-Mata L, Morales R, Reyes-García V, Pardo-de-Santayana M (2014) Weeds and food diversity: natural yield assessment and future alternatives for traditionally consumed wild vegetables. J Ethnopharmacol 34: 44–67.
Nave A. Gonçalves F, Teixeira R, Costa CA, Campos M, Torres L. (2016) Hymenoptera parasitoid complex of Prays oleae (Bernard) (Lepidoptera: Praydidae) in Portugal. Turk J Zool (in press).
Parada M, Carrió E, Vallès J (2011) Ethnobotany of food plants in the Alt Emporda region (Catalonia, Iberian Peninsula). J Appl Bot Food Qual 84: 11–25.
Paredes D, Cayuela L, Gurr GM, Campos M (2013) Effect of non-crop vegetation types on conservation biological control of pests in olive groves. PeerJ 1: e116. doi: 10.7717/peerj.116. pmid:23904994.
Pascual S, Cobos G, Seris E, Gonzalez-Nunez M (2010) Effects of processed kaolin on pests and non-target arthropods in a Spanish olive grove. J Pest Sci 83:121–133.
Perrino EV, Calabrese G (2014) Vascular flora of the ancient olive groves of Apulia (southern Italy). Nat Croat 23: 189–218.
Petanidou T (2005) Sugars in mediterranean floral nectars: an ecological and evolutionary approach. J Chem Ecol 31: 1065-1088.
Pinheiro L, Torres LM, Raimundo J, Santos SAP (2013a) Effect of seven species of the family Asteraceae on longevity and nutrient levels of Episyrphus balteatus. BioControl 58: 797-806.
Pinheiro LA, Torres L, Raimundo J, Santos SAP (2013b) Effect of floral resources on longevity and nutrient levels of Episyrphus balteatus (Diptera, Syrphidae). Biol Control 67: 178–185.
Pinheiro LA, Torres LM, Raimundo J, Santos SAP (2015) Effects of pollen, sugars and honeydew on lifespan and nutrient levels of Episyrphus balteatus. BioControl 60: 47-57.
Pontin, DR, Wade MR, Kehrli P, Wratten SD (2006) Attractiveness of single and multiple species flower patches to beneficial insects in agroecosystems. An Appl Biol 148: 39–47.
Raunkiaer CC (1934) The life forms of plants and statistical plant geography. Oxford University Press.
Rebek EJ, Sadof CS, Hanks LM (2005) Manipulating the abundance of natural enemies in ornamental landscapes with floral resource plants. Biol Control 33: 203–216.
Rivera D, Obón C, Heinrich M, Inocencio C, Verde A, Fajardo J (2006) Gathered mediterranean food plants – ethnobotanical investigations and historical development. In: Heinrich M, Müller WE, Galli C (eds) Local Mediterranean food plants and nutraceuticals. Basel: Karger, (Forum of Nutrition; vol 59) pp. 18–74.
Rogers ME, Potter DA (2004) Potential for sugar sprays and flowering plants to increase parasitism of white grubs (Coleoptera: Scarabaeidae) by Tiphiid wasps (Hymenoptera: Tiphiidae). Environ Entomol 33: 619–626.
Saeed S, Sajjad A (2010) Floral Host Plant Range of Syrphid Flies (Syrphidae: Diptera) Under Natural Conditions in Southern Punjab Pakistan. Pak J Bot 42: 1187-1200.
Shrewsbury PM, Lashomb JH, Hamilton GC, Zhang J, Patt JM, Casagrande RA (2004) The influence of flowering plants on herbivore and natural enemy abundance in ornamental landscapes. J of Ecol and Envi Scie 30: 23– 33.
Sivinski J, Wahl D, Holler T, Al-Dobai S, Sivinski R (2011) Conserving natural enemies with flowering plants; estimating floral attractiveness to parasitic Hymenoptera and attractions correlates to flower and plant morphology. Biol Control 58: 208-214.
Santos SAP, Pereira JA, Torres LM, Nogeira AJA (2009) Voracity of coccinellid species on different phenological stages of the olive pest Saissetia oleae (Hom.: Coccidae). Appl Ecol Environ Res 7: 359– 365.
Tardio J, Pardo-De-Santayana M, Morales R. (2006) Ethnobotanical review of wild edible plants in Spain. Bot J Linn Soc 152: 27–71.
Tschorsnig H-P, Seris E, Cobo A, Cobos G, Pascual S, Ros JP, Gonzalez-Nuñez M (2011) Tachinidae (Diptera) collected in traps used for mass-trapping of Bactrocera oleae (Rossi) (Diptera: Tephritidae) in olive groves in Central Spain. Span J Agric Res 9: 1298-1306.
Tompkins JML, Wratten SD, Wäckers FL (2010) Nectar to improve parasitoid fitness in biological control: does the sucrose: hexose ratio matter? Basic Appl Ecol 11: 264–271.
Viggiani G (1986) La protection phytosanitaire en oleiculture. In Arambourg Y. (Ed) Entomologie oleicole. Conseil Oleicole International, Madrid: 259-347.
Villa M, Santos SAP, Mexia A, Bento A, Pereira JA (2016) Ground cover management affects parasitism of Prays oleae (Bernard). Biol Control 96: 72–77.
Villenave J, Thierry D, Mamun AA, Lodé T, Rat-Morris E (2005) The pollens consumed by common green lacewings Chrysoperla spp. (Neuroptera: Chrysopidae) in cabbage crop environment in western France. Eur J Entomol. 102: 547-552.
Vogiatzakis IN, Mannion AM, Griffiths GH (2006) Mediterranean ecosystems: problems and tools for conservation. Prog Phys Geogr 30: 175–200.
Wäckers FL (2004) Assessing the suitability of flowering herbs as parasitoid food sources: flower attractiveness and nectar accessibility. Biol Control 29, 307–314.
Wade, M.R., Gurr, G.M. and Wratten, S.D. (2008) Ecological restoration of farmland: progress and prospects. Philos Trans R Soc Lond B Biol Sci 363: 831–847.
Winkler K, Wäckers FL, Kaufman LV, Larraz V, van Lenteren JC (2009) Nectar exploitation by herbivores and their parasitoids is a function of flower species and relative humidity. Biol Control 50: 299–306.